Npas1+ Pallidal Neurons Target Striatal Projection Neurons
- PMID: 27194328
- PMCID: PMC4871984
- DOI: 10.1523/JNEUROSCI.1720-15.2016
Npas1+ Pallidal Neurons Target Striatal Projection Neurons
Abstract
Compelling evidence demonstrates that the external globus pallidus (GPe) plays a key role in processing sensorimotor information. An anatomical projection from the GPe to the dorsal striatum has been described for decades. However, the cellular target and functional impact of this projection remain unknown. Using cell-specific transgenic mice, modern monosynaptic tracing techniques, and optogenetics-based mapping, we discovered that GPe neurons provide inhibitory inputs to direct and indirect pathway striatal projection neurons (SPNs). Our results indicate that the GPe input to SPNs arises primarily from Npas1-expressing neurons and is strengthened in a chronic Parkinson's disease (PD) model. Alterations of the GPe-SPN input in a PD model argue for the critical position of this connection in regulating basal ganglia motor output and PD symptomatology. Finally, chemogenetic activation of Npas1-expressing GPe neurons suppresses motor output, arguing that strengthening of the GPe-SPN connection is maladaptive and may underlie the hypokinetic symptoms in PD.
Significance statement: An anatomical projection from the pallidum to the striatum has been described for decades, but little is known about its connectivity pattern. The authors dissect the presynaptic and postsynaptic neurons involved in this projection, and show its cell-specific remodeling and strengthening in parkinsonian mice. Chemogenetic activation of Npas1(+) pallidal neurons that give rise to the principal pallidostriatal projection increases the time that the mice spend motionless. This argues that maladaptive strengthening of this connection underlies the paucity of volitional movements, which is a hallmark of Parkinson's disease.
Keywords: 6-OHDA; Npas1-Cre; arkypallidal neurons; extrinsic inhibition; pallidostriatal projection.
Copyright © 2016 the authors 0270-6474/16/365472-17$15.00/0.
Figures
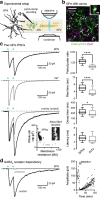

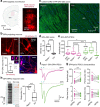
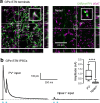

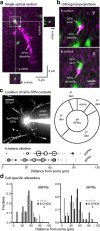
References
-
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci. 2015;35:6667–6688. doi: 10.1523/JNEUROSCI.4662-14.2015. - DOI - PMC - PubMed
-
- Abedi PM, Delaville C, De Deurwaerdère P, Benjelloun W, Benazzouz A. Intrapallidal administration of 6-hydroxydopamine mimics in large part the electrophysiological and behavioral consequences of major dopamine depletion in the rat. Neuroscience. 2013;236:289–297. doi: 10.1016/j.neuroscience.2013.01.043. - DOI - PubMed
-
- Anderson ME, Horak FB. Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol. 1985;54:433–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
