Liposome production by microfluidics: potential and limiting factors
- PMID: 27194474
- PMCID: PMC4872163
- DOI: 10.1038/srep25876
Liposome production by microfluidics: potential and limiting factors
Abstract
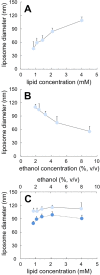
This paper provides an analysis of microfluidic techniques for the production of nanoscale lipid-based vesicular systems. In particular we focus on the key issues associated with the microfluidic production of liposomes. These include, but are not limited to, the role of lipid formulation, lipid concentration, residual amount of solvent, production method (including microchannel architecture), and drug loading in determining liposome characteristics. Furthermore, we propose microfluidic architectures for the mass production of liposomes with a view to potential industrial translation of this technology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Walde P. Preparation of vesicles (liposomes) In Encyclopedia of Nanoscience and Nanotechnology (ed. Nalwa H. S.) 9, 43–79 (Americal Scientific Publishers, 2004).
-
- Fan Y. & Zhang Q. Development of liposomal formulations: From concept to clinical investigations. Asian J. Pharm. Sci. 8, 79–90 (2013).
-
- Allen T. M. & Cullis P. R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013). - PubMed
-
- Herzog C. et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine 27, 4381–4387 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

