Cardiac troponin T is necessary for normal development in the embryonic chick heart
- PMID: 27194630
- PMCID: PMC4974548
- DOI: 10.1111/joa.12486
Cardiac troponin T is necessary for normal development in the embryonic chick heart
Abstract
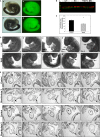
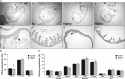
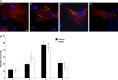
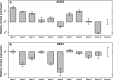
The heart is the first functioning organ to develop during embryogenesis. The formation of the heart is a tightly regulated and complex process, and alterations to its development can result in congenital heart defects. Mutations in sarcomeric proteins, such as alpha myosin heavy chain and cardiac alpha actin, have now been associated with congenital heart defects in humans, often with atrial septal defects. However, cardiac troponin T (cTNT encoded by gene TNNT2) has not. Using gene-specific antisense oligonucleotides, we have investigated the role of cTNT in chick cardiogenesis. TNNT2 is expressed throughout heart development and in the postnatal heart. TNNT2-morpholino treatment resulted in abnormal atrial septal growth and a reduction in the number of trabeculae in the developing primitive ventricular chamber. External analysis revealed the development of diverticula from the ventricular myocardial wall which showed no evidence of fibrosis and still retained a myocardial phenotype. Sarcomeric assembly appeared normal in these treated hearts. In humans, congenital ventricular diverticulum is a rare condition, which has not yet been genetically associated. However, abnormal haemodynamics is known to cause structural defects in the heart. Further, structural defects, including atrial septal defects and congenital diverticula, have previously been associated with conduction anomalies. Therefore, to provide mechanistic insights into the effect that cTNT knockdown has on the developing heart, quantitative PCR was performed to determine the expression of the shear stress responsive gene NOS3 and the conduction gene TBX3. Both genes were differentially expressed compared to controls. Therefore, a reduction in cTNT in the developing heart results in abnormal atrial septal formation and aberrant ventricular morphogenesis. We hypothesize that alterations to the haemodynamics, indicated by differential NOS3 expression, causes these abnormalities in growth in cTNT knockdown hearts. In addition, the muscular diverticula reported here suggest a novel role for mutations of structural sarcomeric proteins in the pathogenesis of congenital cardiac diverticula. From these studies, we suggest TNNT2 is a gene worthy of screening for those with a congenital heart defect, particularly atrial septal defects and ventricular diverticula.
Keywords: atrial septal defects; cardiac troponin T; congenital cardiac diverticula; heart development; structural protein.
© 2016 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures


References
-
- Anderson PA, Malouf NN, Oakeley AE, et al. (1991) Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 69, 1226–1233. - PubMed
-
- Anderson PAW, Greig A, Mark TM, et al. (1995) Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76, 681–686. - PubMed
-
- Bakker ML, Boukens BJ, Mommersteeg MTM, et al. (2008) Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ Res 102, 1340–1349. - PubMed
-
- Baruteau AE, Probst V, Abriel H (2015) Inherited progressive cardiac conduction disorders. Curr Opin Cardiol 30, 33–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

