Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury
- PMID: 27194713
- PMCID: PMC5008672
- DOI: 10.1152/ajprenal.00056.2016
Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury
Abstract
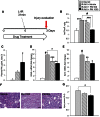
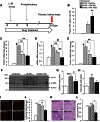
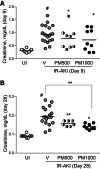
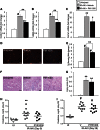
Acute kidney injury (AKI) is a common and independent risk factor for death and chronic kidney disease (CKD). Despite promising preclinical data, there is no evidence that antioxidants reduce the severity of injury, increase recovery, or prevent CKD in patients with AKI. Pyridoxamine (PM) is a structural analog of vitamin B6 that interferes with oxidative macromolecular damage via a number of different mechanisms and is in a phase 3 clinical efficacy trial to delay CKD progression in patients with diabetic kidney disease. Because oxidative stress is implicated as one of the main drivers of renal injury after AKI, the ability of PM to interfere with multiple aspects of oxidative damage may be favorable for AKI treatment. In these studies we therefore evaluated PM treatment in a mouse model of AKI. Pretreatment with PM caused a dose-dependent reduction in acute tubular injury, long-term postinjury fibrosis, as well as improved functional recovery after ischemia-reperfusion AKI (IR-AKI). This was associated with a dose-dependent reduction in the oxidative stress marker isofuran-to-F2-isoprostane ratio, indicating that PM reduces renal oxidative damage post-AKI. PM also reduced postinjury fibrosis when administered 24 h after the initiating injury, but this was not associated with improvement in functional recovery after IR-AKI. This is the first report showing that treatment with PM reduces short- and long-term injury, fibrosis, and renal functional recovery after IR-AKI. These preclinical findings suggest that PM, which has a favorable clinical safety profile, holds therapeutic promise for AKI and, most importantly, for prevention of adverse long-term outcomes after AKI.
Keywords: oxidative stress; renal fibrosis; renal function.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Alderson NL, Chachich ME, Youssef NN, Beattie RJ, Nachtigal M, Thorpe SR, Baynes JW. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int 63: 2123–2133, 2003. - PubMed
-
- Arora MK, Singh UK. Oxidative stress: meeting multiple targets in pathogenesis of diabetic nephropathy. Curr Drug Targets 15: 531–538, 2014. - PubMed
-
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

