The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction
- PMID: 27194714
- PMCID: PMC4967168
- DOI: 10.1152/ajprenal.00164.2016
The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction
Abstract
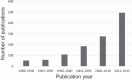
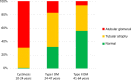
There is an alarming global increase in the incidence of end-stage kidney disease, for which early biomarkers and effective treatment options are lacking. Largely based on the histology of the end-stage kidney and on the model of unilateral ureteral obstruction, current investigation is focused on the pathogenesis of renal interstitial fibrosis as a central mechanism in the progression of chronic kidney disease (CKD). It is now recognized that cumulative episodes of acute kidney injury (AKI) can lead to CKD, and, conversely, CKD is a risk factor for AKI. Based on recent and historic studies, this review shifts attention from the glomerulus and interstitium to the proximal tubule as the primary sensor and effector in the progression of CKD as well as AKI. Packed with mitochondria and dependent on oxidative phosphorylation, the proximal tubule is particularly vulnerable to injury (obstructive, ischemic, hypoxic, oxidative, metabolic), resulting in cell death and ultimately in the formation of atubular glomeruli. Animal models of human glomerular and tubular disorders have provided evidence for a broad repertoire of morphological and functional responses of the proximal tubule, revealing processes of degeneration and repair that may lead to new therapeutic strategies. Most promising are studies that encompass the entire life cycle from fetus to senescence, recognizing epigenetic factors. The application of techniques in molecular characterization of tubule segments and the development of human kidney organoids may provide new insights into the mammalian kidney subjected to stress or injury, leading to biomarkers of early CKD and new therapies.
Keywords: acute kidney injury; atubular glomeruli; chronic kidney disease; proximal tubule; unilateral ureteral obstruction.
Copyright © 2016 the American Physiological Society.
Figures




References
-
- Adam B, Mengel M. Molecular nephropathology: ready for prime time? Am J Physiol Renal Physiol 309: F185–F188, 2015. - PubMed
-
- Addis T, Oliver J. The Renal Lesion in Bright's Disease. New York: Paul Hoeber, 1931.
-
- Arendshorst WJ, Finn WF, Gottschalk CW. Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res 37: 558–568, 1975. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

