Grafted Subventricular Zone Neural Stem Cells Display Robust Engraftment and Similar Differentiation Properties and Form New Neurogenic Niches in the Young and Aged Hippocampus
- PMID: 27194744
- PMCID: PMC4996439
- DOI: 10.5966/sctm.2015-0270
Grafted Subventricular Zone Neural Stem Cells Display Robust Engraftment and Similar Differentiation Properties and Form New Neurogenic Niches in the Young and Aged Hippocampus
Abstract
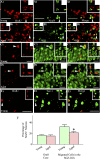
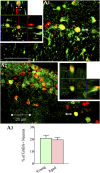
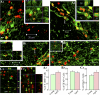
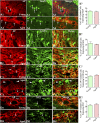
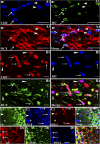
: As clinical application of neural stem cell (NSC) grafting into the brain would also encompass aged people, critical evaluation of engraftment of NSC graft-derived cells in the aged hippocampus has significance. We examined the engraftment and differentiation of alkaline phosphatase-positive NSCs expanded from the postnatal subventricular zone (SVZ), 3 months after grafting into the intact young or aged rat hippocampus. Graft-derived cells engrafted robustly into both young and aged hippocampi. Although most graft-derived cells pervasively migrated into different hippocampal layers, the graft cores endured and contained graft-derived neurons expressing neuron-specific nuclear antigen (NeuN) and γ-amino butyric acid in both groups. A fraction of migrated graft-derived cells in the neurogenic subgranular zone-granule cell layer also expressed NeuN. Neuronal differentiation was, however, occasionally seen amid graft-derived cells that had migrated into non-neurogenic regions, where substantial fractions differentiated into S-100β+ astrocytes, NG2+ oligodendrocyte progenitors, or Olig2+ putative oligodendrocytes. In both age groups, graft cores located in non-neurogenic regions displayed many doublecortin-positive (DCX+) immature neurons at 3 months after grafting. Analyses of cells within graft cores using birth dating and putative NSC markers revealed that DCX+ neurons were newly born neurons derived from engrafted cells and that putative NSCs persisted within the graft cores. Thus, both young and aged hippocampi support robust engraftment and similar differentiation of SVZ-NSC graft-derived cells. Furthermore, some grafted NSCs retain the "stemness" feature and produce new neurons even at 3 months after grafting, implying that grafting of SVZ-NSCs into the young or aged hippocampus leads to establishment of new neurogenic niches in non-neurogenic regions.
Significance: The results demonstrate that advanced age of the host at the time of grafting has no major adverse effects on engraftment, migration, and differentiation of grafted subventricular zone-neural stem cells (SVZ-NSCs) in the intact hippocampus, as both young and aged hippocampi promoted excellent engraftment, migration, and differentiation of SVZ-NSC graft-derived cells in the present study. Furthermore, SVZ-NSC grafts showed ability for establishing neurogenic niches in non-neurogenic regions, generating new neurons for extended periods after grafting. This phenomenon will be beneficial if these niches can continuously generate new neurons and glia in the grafted hippocampus, as newly generated neurons and glia are expected to improve, not only the microenvironment, but also the plasticity and function of the aged hippocampus. Overall, these results have significance because the potential application of NSC grafting for treatment of neurodegenerative disorders at early stages of disease progression and age-related impairments would mostly involve aged persons as recipients.
Keywords: Cell transplantation; Cellular therapy; Hippocampal neurogenesis; Neural stem cells; Stem cell grafts; Subventricular zone stem cells.
©AlphaMed Press.
Figures


Similar articles
-
Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus.Stem Cells. 2007 Aug;25(8):2104-17. doi: 10.1634/stemcells.2006-0726. Epub 2007 May 17. Stem Cells. 2007. PMID: 17510219
-
Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis.Stem Cells Transl Med. 2012 Sep;1(9):696-708. doi: 10.5966/sctm.2012-0050. Epub 2012 Sep 5. Stem Cells Transl Med. 2012. PMID: 23197876 Free PMC article.
-
Identification of NeuN immunopositive cells in the adult mouse subventricular zone.J Comp Neurol. 2018 Aug 15;526(12):1927-1942. doi: 10.1002/cne.24463. J Comp Neurol. 2018. PMID: 29752725
-
The vasculature of neurogenic niches: Properties and function.Cells Dev. 2023 Jun;174:203841. doi: 10.1016/j.cdev.2023.203841. Epub 2023 Apr 14. Cells Dev. 2023. PMID: 37060947 Review.
-
The ventricular-subventricular, subgranular and subcallosal zones: three niches of neural stem cells in the postnatal brain.Exp Brain Res. 2023 Jun;241(6):1463-1470. doi: 10.1007/s00221-023-06621-w. Epub 2023 Apr 21. Exp Brain Res. 2023. PMID: 37083843 Review.
Cited by
-
Acupuncture Improves Cerebral Microenvironment in Mice with Alzheimer's Disease Treated with Hippocampal Neural Stem Cells.Mol Neurobiol. 2017 Sep;54(7):5120-5130. doi: 10.1007/s12035-016-0054-5. Epub 2016 Aug 24. Mol Neurobiol. 2017. PMID: 27558235
-
Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration.Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):287-296. doi: 10.1073/pnas.1814185115. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559206 Free PMC article.
-
Human induced neural stem cells support functional recovery in spinal cord injury models.Exp Mol Med. 2023 Jun;55(6):1182-1192. doi: 10.1038/s12276-023-01003-2. Epub 2023 Jun 1. Exp Mol Med. 2023. PMID: 37258581 Free PMC article.
-
Chronobiology of limbic seizures: Potential mechanisms and prospects of chronotherapy for mesial temporal lobe epilepsy.Neurosci Biobehav Rev. 2019 Mar;98:122-134. doi: 10.1016/j.neubiorev.2019.01.004. Epub 2019 Jan 7. Neurosci Biobehav Rev. 2019. PMID: 30629979 Free PMC article. Review.
-
Adult Neural Stem Cells from Midbrain Periventricular Regions Show Limited Neurogenic Potential after Transplantation into the Hippocampal Neurogenic Niche.Cells. 2021 Nov 4;10(11):3021. doi: 10.3390/cells10113021. Cells. 2021. PMID: 34831242 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

