Experimental Anti-Inflammatory Drug Semapimod Inhibits TLR Signaling by Targeting the TLR Chaperone gp96
- PMID: 27194788
- PMCID: PMC4896225
- DOI: 10.4049/jimmunol.1502135
Experimental Anti-Inflammatory Drug Semapimod Inhibits TLR Signaling by Targeting the TLR Chaperone gp96
Abstract
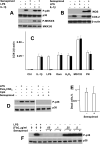
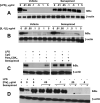
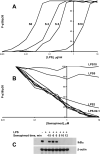
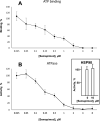
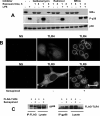
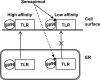
Semapimod, a tetravalent guanylhydrazone, suppresses inflammatory cytokine production and has potential in a variety of inflammatory and autoimmune disorders. The mechanism of action of Semapimod is not well understood. In this study, we demonstrate that in rat IEC-6 intestinal epithelioid cells, Semapimod inhibits activation of p38 MAPK and NF-κB and induction of cyclooxygenase-2 by TLR ligands, but not by IL-1β or stresses. Semapimod inhibits TLR4 signaling (IC50 ≈0.3 μmol) and acts by desensitizing cells to LPS; it fails to block responses to LPS concentrations of ≥5 μg/ml. Inhibition of TLR signaling by Semapimod is almost instantaneous: the drug is effective when applied simultaneously with LPS. Semapimod blocks cell-surface recruitment of the MyD88 adapter, one of the earliest events in TLR signaling. gp96, the endoplasmic reticulum-localized chaperone of the HSP90 family critically involved in the biogenesis of TLRs, was identified as a target of Semapimod using ATP-desthiobiotin pulldown and mass spectroscopy. Semapimod inhibits ATP-binding and ATPase activities of gp96 in vitro (IC50 ≈0.2-0.4 μmol). On prolonged exposure, Semapimod causes accumulation of TLR4 and TLR9 in perinuclear space, consistent with endoplasmic reticulum retention, an anticipated consequence of impaired gp96 chaperone function. Our data indicate that Semapimod desensitizes TLR signaling via its effect on the TLR chaperone gp96. Fast inhibition by Semapimod is consistent with gp96 participating in high-affinity sensing of TLR ligands in addition to its role as a TLR chaperone.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures


Similar articles
-
Gp96 deficiency affects TLR4 functionality and impairs ERK and p38 phosphorylation.PLoS One. 2018 Feb 15;13(2):e0193003. doi: 10.1371/journal.pone.0193003. eCollection 2018. PLoS One. 2018. PMID: 29447283 Free PMC article.
-
Toll-like receptor 4-mediated c-Jun N-terminal kinase activation induces gp96 cell surface expression via AIMP1 phosphorylation.Biochem Biophys Res Commun. 2010 Jun 18;397(1):100-5. doi: 10.1016/j.bbrc.2010.05.075. Epub 2010 May 16. Biochem Biophys Res Commun. 2010. PMID: 20510162
-
Propofol Inhibits Lipopolysaccharide-Induced Inflammatory Responses in Spinal Astrocytes via the Toll-Like Receptor 4/MyD88-Dependent Nuclear Factor-κB, Extracellular Signal-Regulated Protein Kinases1/2, and p38 Mitogen-Activated Protein Kinase Pathways.Anesth Analg. 2015 Jun;120(6):1361-8. doi: 10.1213/ANE.0000000000000645. Anesth Analg. 2015. PMID: 25695672
-
Roles of heat shock protein gp96 in the ER quality control: redundant or unique function?Mol Cells. 2005 Oct 31;20(2):173-82. Mol Cells. 2005. PMID: 16267390 Review.
-
Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94.Curr Top Med Chem. 2016;16(25):2765-78. doi: 10.2174/1568026616666160413141613. Curr Top Med Chem. 2016. PMID: 27072698 Free PMC article. Review.
Cited by
-
Ligustrazine Alleviate Acute Lung Injury Through Suppressing Pyroptosis and Apoptosis of Alveolar Macrophages.Front Pharmacol. 2021 May 28;12:680512. doi: 10.3389/fphar.2021.680512. eCollection 2021. Front Pharmacol. 2021. PMID: 34122107 Free PMC article.
-
Discovery and optimization of a guanylhydrazone-based small molecule to replace bFGF for cell culture applications.Biochem Biophys Rep. 2025 Jul 21;43:102167. doi: 10.1016/j.bbrep.2025.102167. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40734998 Free PMC article.
-
Regulation of dendritic cell function improves survival in experimental sepsis through immune chaperone.Innate Immun. 2019 May;25(4):235-243. doi: 10.1177/1753425919840423. Innate Immun. 2019. PMID: 31018807 Free PMC article.
-
Rapid Natural Killer Cell Gene Responses, Generated by TLR Ligand-Induced Trained Immunity, Provide Protection to Bacterial Infection in rag1-/- Mutant Zebrafish (Danio rerio).Int J Mol Sci. 2025 Jan 23;26(3):962. doi: 10.3390/ijms26030962. Int J Mol Sci. 2025. PMID: 39940731 Free PMC article.
-
Discovery of antibiotics that selectively kill metabolically dormant bacteria.Cell Chem Biol. 2024 Apr 18;31(4):712-728.e9. doi: 10.1016/j.chembiol.2023.10.026. Epub 2023 Nov 28. Cell Chem Biol. 2024. PMID: 38029756 Free PMC article.
References
-
- Bianchi M, Ulrich P, Bloom O, Meistrell M, 3rd, Zimmerman GA, Schmidtmayerova H, Bukrinsky M, Donnelley T, Bucala R, Sherry B, et al. An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality. Mol. Med. 1995;1:254–266. - PMC - PubMed
-
- Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, Rauvala H, Palmblad J, Andersson J, Treutiger CJ. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand. J. Immunol. 2004;60:566–573. - PubMed
-
- Zinser E, Turza N, Steinkasserer A. CNI-1493 mediated suppression of dendritic cell activation in vitro and in vivo. Immunobiology. 2004;209:89–97. - PubMed
-
- Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J. Immunol. 2006;176:580–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

