Big insights from small volumes: deciphering complex leukocyte behaviors using microfluidics
- PMID: 27194799
- PMCID: PMC4945356
- DOI: 10.1189/jlb.5RU0216-056R
Big insights from small volumes: deciphering complex leukocyte behaviors using microfluidics
Abstract
Inflammation is an indispensable component of the immune response, and leukocytes provide the first line of defense against infection. Although the major stereotypic leukocyte behaviors in response to infection are well known, the complexities and idiosyncrasies of these phenotypes in conditions of disease are still emerging. Novel tools are indispensable for gaining insights into leukocyte behavior, and in the past decade, microfluidic technologies have emerged as an exciting development in the field. Microfluidic devices are readily customizable, provide tight control of experimental conditions, enable high precision of ex vivo measurements of individual as well as integrated leukocyte functions, and have facilitated the discovery of novel leukocyte phenotypes. Here, we review some of the most interesting insights resulting from the application of microfluidic approaches to the study of the inflammatory response. The aim is to encourage leukocyte biologists to integrate these new tools into increasingly more sophisticated experimental designs for probing complex leukocyte functions.
Keywords: dendritic cells; inflammation; monocytes; nanotechnology; neutrophils.
© Society for Leukocyte Biology.
Figures
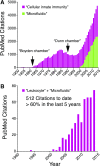



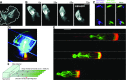
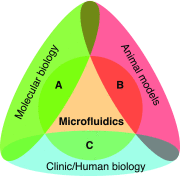
Similar articles
-
High-throughput screening approaches and combinatorial development of biomaterials using microfluidics.Acta Biomater. 2016 Apr 1;34:1-20. doi: 10.1016/j.actbio.2015.09.009. Epub 2015 Sep 8. Acta Biomater. 2016. PMID: 26361719 Review.
-
Droplet-based microfluidics: enabling impact on drug discovery.J Biomol Screen. 2014 Apr;19(4):483-96. doi: 10.1177/1087057113510401. Epub 2013 Nov 15. J Biomol Screen. 2014. PMID: 24241711 Review.
-
Analysis of Leukocyte Behaviors on Microfluidic Chips.Adv Healthc Mater. 2019 Feb;8(4):e1801406. doi: 10.1002/adhm.201801406. Epub 2019 Jan 23. Adv Healthc Mater. 2019. PMID: 30672149 Review.
-
Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20560-5. doi: 10.1073/pnas.1210269109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23185003 Free PMC article.
-
Vascular mimetics based on microfluidics for imaging the leukocyte--endothelial inflammatory response.Lab Chip. 2007 Apr;7(4):448-56. doi: 10.1039/b617915k. Epub 2007 Jan 23. Lab Chip. 2007. PMID: 17389960
Cited by
-
Microfluidic Systems to Study Neutrophil Forward and Reverse Migration.Front Immunol. 2021 Nov 24;12:781535. doi: 10.3389/fimmu.2021.781535. eCollection 2021. Front Immunol. 2021. PMID: 34899746 Free PMC article. Review.
-
Rapid antibiotic sensitivity testing in microwell arrays.Technology (Singap World Sci). 2017 Jun;5(2):107-114. doi: 10.1142/S2339547817500030. Epub 2017 May 16. Technology (Singap World Sci). 2017. PMID: 28781994 Free PMC article.
-
Profiling migration of human monocytes in response to chemotactic and barotactic guidance cues.Cell Rep Methods. 2024 Sep 16;4(9):100846. doi: 10.1016/j.crmeth.2024.100846. Epub 2024 Sep 5. Cell Rep Methods. 2024. PMID: 39241776 Free PMC article.
-
Neutrophil Chemotaxis in One Droplet of Blood Using Microfluidic Assays.Methods Mol Biol. 2018;1749:351-360. doi: 10.1007/978-1-4939-7701-7_25. Methods Mol Biol. 2018. PMID: 29526009 Free PMC article.
-
Outer Membrane Structural Defects in Salmonella enterica Serovar Typhimurium Affect Neutrophil Chemokinesis but Not Chemotaxis.mSphere. 2021 Feb 24;6(1):e01012-20. doi: 10.1128/mSphere.01012-20. mSphere. 2021. PMID: 33627508 Free PMC article.
References
-
- Meira L. B., Bugni J. M., Green S. L., Lee C. W., Pang B., Borenshtein D., Rickman B. H., Rogers A. B., Moroski-Erkul C. A., McFaline J. L., Schauer D. B., Dedon P. C., Fox J. G., Samson L. D. (2008) DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 118, 2516–2525. - PMC - PubMed
-
- Komohara Y., Fujiwara Y., Ohnishi K., Takeya M. (2015) Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 99, 180–185. - PubMed
-
- Willerson J. T., Ridker P. M. (2004) Inflammation as a cardiovascular risk factor. Circulation 109 (21 Suppl 1), II2–II10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

