Tricellular Tight Junctions in the Inner Ear
- PMID: 27195292
- PMCID: PMC4852330
- DOI: 10.1155/2016/6137541
Tricellular Tight Junctions in the Inner Ear
Abstract
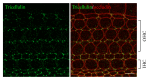
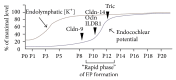
Tight junctions (TJs) are structures that seal the space between the epithelial cell sheets. In the inner ear, the barrier function of TJs is indispensable for the separation of the endolymphatic and perilymphatic spaces, which is essential for the generation and maintenance of the endocochlear potential (EP). TJs are formed by the intercellular binding of membrane proteins, known as claudins, and mutations in these proteins cause deafness in humans and mice. Within the epithelial cell sheet, however, a bound structure is present at the site where the corners of three cells meet (tricellular tight junctions (tTJs)), and the maintenance of the barrier function at this location cannot be explained by the claudins alone. Tricellulin and the angulin family of proteins (angulin-1/LSR, angulin-2/ILDR1, and angulin-3/ILDR2) have been identified as tTJ-associated proteins. Tricellulin and ILDR1 are localized at the tTJ and alterations in these proteins have been reported to be involved in deafness. In this review, we will present the current state of knowledge for tTJs.
Figures

References
-
- Ferrary E., Sterkers O. Mechanisms of endolymph secretion. Kidney International. 1998;65:S98–S103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

