Population pharmacokinetics and prophylactic anti-emetic efficacy of ramosetron in surgical patients
- PMID: 27195435
- PMCID: PMC5338108
- DOI: 10.1111/bcp.13010
Population pharmacokinetics and prophylactic anti-emetic efficacy of ramosetron in surgical patients
Abstract
Aims: This study characterized the pharmacokinetics of ramosetron and compared prophylactic anti-emetic efficacy with that of ondansetron in a large population.
Methods: Fifty-eight patients consented to the pharmacokinetic analysis and were assigned randomly to receive 0.3, 0.45 or 0.6 mg ramosetron after induction of anaesthesia. Blood samples were acquired at preset intervals. Non-compartmental and population pharmacokinetic analyses were performed. In total, 1102 patients consented to the evaluation of prophylactic anti-emetic efficacy and were allocated randomly to receive 0.3 mg ramosetron or 4 mg ondansetron at the end of surgery. An additional 16 mg ondansetron were mixed in the intravenous patient-controlled analgesia pump of the ondansetron group. Post-operative nausea and vomiting (PONV) were evaluated 6, 24 and 48 h post-operatively using the Rhodes index of nausea, vomiting and retching (RINVR). Administration of rescue anti-emetics and adverse events were evaluated.
Results: The pharmacokinetic parameter estimates were V1 (l) = 5.12, V2 (l) = 108, CL (l⋅min(-1) ) = 0.08 + (59⋅age(-1) ) × 0.09, Q (l⋅min(-1) ) = 1.42. The incidences of PONV in the ramosetron and ondansetron groups were 77 (13.9%) and 113 (20.6%) and 44 (7.9%) and 66 (12.0%) at 24 and 48 h post-operatively, respectively (P = 0.004, 0.030). RINVR was significantly lower in the ramosetron than the ondansetron group 24 and 48 h post-operatively (P = 0.003, 0.025). Use of rescue anti-emetics and incidence of adverse events were comparable.
Conclusions: A two compartment mammillary model was used to describe ramosetron pharmacokinetics. Prophylactic anti-emetic efficacy of ramosetron was significantly better 24 and 48 h post-operatively than that of ondansetron, particularly when the Apfel score was ≥ 3.
Keywords: anti-emetics; pharmacokinetics; post-operative nausea and vomiting; ramosetron.
© 2016 The British Pharmacological Society.
Figures
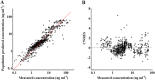
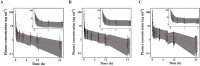

 ramosetron,
ramosetron,  ondansetron
ondansetronReferences
-
- Gan T, Sloan F, Dear Gde L, El‐Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg 2001; 92: 393–400. - PubMed
-
- Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 1999; 89: 652–8. - PubMed
-
- Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross‐validations between two centers. Anesthesiology 1999; 91: 693–700. - PubMed
-
- Gan TJ, Ginsberg B, Grant AP, Glass PS. Double‐blind, randomized comparison of ondansetron and intraoperative propofol to prevent postoperative nausea and vomiting. Anesthesiology 1996; 85: 1036–42. - PubMed
-
- Habib AS, Gan TJ. Evidence‐based management of postoperative nausea and vomiting: a review. Can J Anaesth 2004; 51: 326–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

