Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing
- PMID: 27195722
- PMCID: PMC4872602
- DOI: 10.1038/srep26227
Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing
Abstract
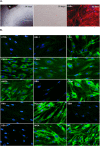
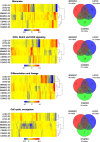
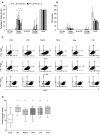
Corneal tissue regeneration is of crucial importance for maintaining normal vision. We aimed to isolate and cultivate human corneal stroma-derived mesenchymal stem-like cells (CSMSCs) from the central part of cadaver corneas and study their phenotype, multipotency, role in immunity and wound healing. The isolated cells grew as monolayers in vitro, expressed mesenchymal- and stemness-related surface markers (CD73, CD90, CD105, CD140b), and were negative for hematopoietic markers as determined by flow cytometry. CSMSCs were able to differentiate in vitro into fat, bone and cartilage. Their gene expression profile was closer to bone marrow-derived MSCs (BMMSCs) than to limbal epithelial stem cells (LESC) as determined by high-throughput screening. The immunosuppressive properties of CSMSCs were confirmed by a mixed lymphocyte reaction (MLR), while they could inhibit proliferation of activated immune cells. Treatment of CSMSCs by pro-inflammatory cytokines and toll-like receptor ligands significantly increased the secreted interleukin-6 (IL-6), interleukin-8 (IL-8) and C-X-C motif chemokine 10 (CXCL-10) levels, as well as the cell surface adhesion molecules. CSMSCs were capable of closing a wound in vitro under different stimuli. These cells thus contribute to corneal tissue homeostasis and play an immunomodulatory and regenerative role with possible implications in future cell therapies for treating sight-threatening corneal diseases.
Figures


Similar articles
-
Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury.Exp Eye Res. 2016 Oct;151:227-35. doi: 10.1016/j.exer.2016.08.011. Epub 2016 Aug 25. Exp Eye Res. 2016. PMID: 27567556
-
Stemness and Regenerative Potential of Corneal Stromal Stem Cells and Their Secretome After Long-Term Storage: Implications for Ocular Regeneration.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3728-3738. doi: 10.1167/iovs.18-23824. Invest Ophthalmol Vis Sci. 2018. PMID: 30046814 Free PMC article.
-
Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface.Exp Eye Res. 2013 Nov;116:312-23. doi: 10.1016/j.exer.2013.10.002. Epub 2013 Oct 18. Exp Eye Res. 2013. PMID: 24145108
-
Progress in corneal wound healing.Prog Retin Eye Res. 2015 Nov;49:17-45. doi: 10.1016/j.preteyeres.2015.07.002. Epub 2015 Jul 18. Prog Retin Eye Res. 2015. PMID: 26197361 Free PMC article. Review.
-
Concise review: Stem cells in the corneal stroma.Stem Cells. 2012 Jun;30(6):1059-63. doi: 10.1002/stem.1100. Stem Cells. 2012. PMID: 22489057 Free PMC article. Review.
Cited by
-
Laminin-511-E8 promotes efficient in vitro expansion of human limbal melanocytes.Sci Rep. 2020 Jul 6;10(1):11074. doi: 10.1038/s41598-020-68120-0. Sci Rep. 2020. PMID: 32632213 Free PMC article.
-
Efficient Isolation and Functional Characterization of Niche Cells from Human Corneal Limbus.Int J Mol Sci. 2022 Mar 2;23(5):2750. doi: 10.3390/ijms23052750. Int J Mol Sci. 2022. PMID: 35269891 Free PMC article.
-
PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche.Cells. 2023 Jan 23;12(3):400. doi: 10.3390/cells12030400. Cells. 2023. PMID: 36766742 Free PMC article.
-
Extracellular Vesicle MicroRNAs From Corneal Stromal Stem Cell Enhance Stemness of Limbal Epithelial Stem Cells by Targeting the Notch Pathway.Invest Ophthalmol Vis Sci. 2023 Sep 1;64(12):42. doi: 10.1167/iovs.64.12.42. Invest Ophthalmol Vis Sci. 2023. PMID: 37768272 Free PMC article.
-
Anti-inflammatory potential of human corneal stroma-derived stem cells determined by a novel in vitro corneal epithelial injury model.World J Stem Cells. 2019 Feb 26;11(2):84-99. doi: 10.4252/wjsc.v11.i2.84. World J Stem Cells. 2019. PMID: 30842807 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

