Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial
- PMID: 27195814
- PMCID: PMC4988796
- DOI: 10.1001/jama.2016.7050
Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial
Abstract
Importance: The appropriate treatment target for systolic blood pressure (SBP) in older patients with hypertension remains uncertain.
Objective: To evaluate the effects of intensive (<120 mm Hg) compared with standard (<140 mm Hg) SBP targets in persons aged 75 years or older with hypertension but without diabetes.
Design, setting, and participants: A multicenter, randomized clinical trial of patients aged 75 years or older who participated in the Systolic Blood Pressure Intervention Trial (SPRINT). Recruitment began on October 20, 2010, and follow-up ended on August 20, 2015.
Interventions: Participants were randomized to an SBP target of less than 120 mm Hg (intensive treatment group, n = 1317) or an SBP target of less than 140 mm Hg (standard treatment group, n = 1319).
Main outcomes and measures: The primary cardiovascular disease outcome was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes. All-cause mortality was a secondary outcome.
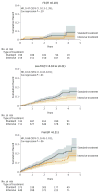
Results: Among 2636 participants (mean age, 79.9 years; 37.9% women), 2510 (95.2%) provided complete follow-up data. At a median follow-up of 3.14 years, there was a significantly lower rate of the primary composite outcome (102 events in the intensive treatment group vs 148 events in the standard treatment group; hazard ratio [HR], 0.66 [95% CI, 0.51-0.85]) and all-cause mortality (73 deaths vs 107 deaths, respectively; HR, 0.67 [95% CI, 0.49-0.91]). The overall rate of serious adverse events was not different between treatment groups (48.4% in the intensive treatment group vs 48.3% in the standard treatment group; HR, 0.99 [95% CI, 0.89-1.11]). Absolute rates of hypotension were 2.4% in the intensive treatment group vs 1.4% in the standard treatment group (HR, 1.71 [95% CI, 0.97-3.09]), 3.0% vs 2.4%, respectively, for syncope (HR, 1.23 [95% CI, 0.76-2.00]), 4.0% vs 2.7% for electrolyte abnormalities (HR, 1.51 [95% CI, 0.99-2.33]), 5.5% vs 4.0% for acute kidney injury (HR, 1.41 [95% CI, 0.98-2.04]), and 4.9% vs 5.5% for injurious falls (HR, 0.91 [95% CI, 0.65-1.29]).
Conclusions and relevance: Among ambulatory adults aged 75 years or older, treating to an SBP target of less than 120 mm Hg compared with an SBP target of less than 140 mm Hg resulted in significantly lower rates of fatal and nonfatal major cardiovascular events and death from any cause.
Trial registration: clinicaltrials.gov Identifier: NCT01206062.
Conflict of interest statement
Figures

Comment in
-
SPRINT Results in Older Patients: How Low to Go?JAMA. 2016 Jun 28;315(24):2669-70. doi: 10.1001/jama.2016.7070. JAMA. 2016. PMID: 27195462 No abstract available.
-
Hypertension: Blood pressure targets for elderly patients: new SPRINT data.Nat Rev Nephrol. 2016 Jul 18;12(8):450-2. doi: 10.1038/nrneph.2016.96. Nat Rev Nephrol. 2016. PMID: 27425394 No abstract available.
-
In adults ≥ 75 years of age with hypertension, intensive vs standard BP-lowering treatment reduced CV events.Ann Intern Med. 2016 Aug 16;165(4):JC14. doi: 10.7326/ACPJC-2016-165-4-014. Ann Intern Med. 2016. PMID: 27538175 No abstract available.
-
Intensive vs Standard Blood Pressure Control for Older Adults.JAMA. 2016 Nov 8;316(18):1921. doi: 10.1001/jama.2016.14909. JAMA. 2016. PMID: 27825001 No abstract available.
-
Intensive vs Standard Blood Pressure Control for Older Adults.JAMA. 2016 Nov 8;316(18):1920-1921. doi: 10.1001/jama.2016.14912. JAMA. 2016. PMID: 27825002 No abstract available.
-
Intensive vs Standard Blood Pressure Control for Older Adults.JAMA. 2016 Nov 8;316(18):1921-1922. doi: 10.1001/jama.2016.14921. JAMA. 2016. PMID: 27825003 No abstract available.
-
Intensive vs Standard Blood Pressure Control for Older Adults.JAMA. 2016 Nov 8;316(18):1922-1923. doi: 10.1001/jama.2016.14927. JAMA. 2016. PMID: 27825004 No abstract available.
-
Intensive treatment of hypertension to a SBP <120 mm Hg in patients aged 75 and over reduces mortality and cardiovascular events.Evid Based Med. 2017 Mar;22(1):30. doi: 10.1136/ebmed-2016-110536. Epub 2016 Nov 21. Evid Based Med. 2017. PMID: 27872159 No abstract available.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. - PubMed
-
- Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277(9):728–734. - PubMed
-
- den Ouden MEM, Schuurmans MJ, Mueller-Schotte S, Bots ML, van der Schouw Y. Do subclinical vascular abnormalities precede impaired physical ability and ADL disability? Exp Gerontol. 2014;58:1–7. - PubMed
-
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR001070/TR/NCATS NIH HHS/United States
- UL1TR000050/TR/NCATS NIH HHS/United States
- HHSN268200900046C/PHS HHS/United States
- 9U54TR000017-06/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- UL1TR000093/TR/NCATS NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1TR000073/TR/NCATS NIH HHS/United States
- UL1RR025755/RR/NCRR NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1RR025771/RR/NCRR NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1TR000433/TR/NCATS NIH HHS/United States
- HHSN268200900040C/PHS HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1TR000105-05/TR/NCATS NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- UL1RR025752/RR/NCRR NIH HHS/United States
- HHSN268200900048C/PHS HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1TR000005/TR/NCATS NIH HHS/United States
- A-HL-13-002-001/HL/NHLBI NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- 01-HL10741/HL/NHLBI NIH HHS/United States
- R01 HL107241/HL/NHLBI NIH HHS/United States
- P30GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- P30-AG21332/AG/NIA NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- R01 HL130500/HL/NHLBI NIH HHS/United States
- HHSN268200900049C/PHS HHS/United States
- UL1TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- HHSN268200900047C/PHS HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1TR001064/TR/NCATS NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- R01 HL107257/HL/NHLBI NIH HHS/United States
- UL1TR000075/TR/NCATS NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

