Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic
- PMID: 27196006
- PMCID: PMC4994725
- DOI: 10.1080/21645515.2016.1157240
Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic
Erratum in
-
Erratum.Hum Vaccin Immunother. 2016 Jul 2;12(7):1948. doi: 10.1080/21645515.2016.1202707. Hum Vaccin Immunother. 2016. PMID: 27398834 Free PMC article. No abstract available.
Abstract
Background: There has been considerable uptake of seasonal influenza vaccines in the Americas compared to other regions. We describe the current influenza vaccination target groups, recent progress in vaccine uptake and in generating evidence on influenza seasonality and vaccine effectiveness for immunization programs. We also discuss persistent challenges, 5 years after the A(H1N1) 2009 influenza pandemic.
Methods: We compiled and summarized data annually reported by countries to the Pan American Health Organization/World Health Organization (PAHO/WHO) through the WHO/UNICEF joint report form on immunization, information obtained through PAHO's Revolving Fund for Vaccine Procurement and communications with managers of national Expanded Programs on Immunization (EPI).
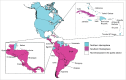
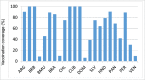
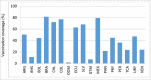
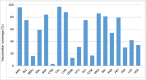
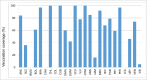
Results: Since 2008, 25 countries/territories in the Americas have introduced new target groups for vaccination or expanded the age ranges of existing target groups. As of 2014, 40 (89%) out of 45 countries/territories have policies established for seasonal influenza vaccination. Currently, 29 (64%) countries/territories target pregnant women for vaccination, the highest priority group according to WHO´s Stategic Advisory Group of Experts and PAHO/WHO's Technical Advisory Group on Vaccine-preventable Diseases, compared to only 7 (16%) in 2008. Among 23 countries reporting coverage data, on average, 75% of adults ≥60 years, 45% of children aged 6-23 months, 32% of children aged 5-2 years, 59% of pregnant women, 78% of healthcare workers, and 90% of individuals with chronic conditions were vaccinated during the 2013-14 Northern Hemisphere or 2014 Southern Hemisphere influenza vaccination activities. Difficulties however persist in the estimation of vaccination coverage, especially for pregnant women and persons with chronic conditions. Since 2007, 6 tropical countries have changed their vaccine formulation from the Northern to the Southern Hemisphere formulation and the timing of their campaigns to April-May following the review of national evidence. LAC countries have also established an official network dedicated to evaluating influenza vaccines effectiveness and impact.
Conclusion: Following the A(H1N1)2009 influenza pandemic, countries of the Americas have continued their efforts to sustain or increase seasonal influenza vaccine uptake among high risk groups, especially among pregnant women. Countries also continued strengthening influenza surveillance, immunization platforms and information systems, indirectly improving preparedness for future pandemics. Influenza vaccination is particularly challenging compared to other vaccines included in EPI schedules, due to the need for annual, optimally timed vaccination, the wide spectrum of target groups, and the limitations of the available vaccines. Countries should continue to monitor influenza vaccination coverage, generate evidence for vaccination programs and implement social communication strategies addressing existing gaps.
Keywords: Americas; Latin America and the Caribbean; immunization; influenza vaccines; seasonal influenza.
Figures
References
-
- World Health Organization Prevention and control of influenza pandemics and annual epidemics Fifty-sixth World Health Assembly, Resolution WHA56.19.28. Geneva: 2003. Available from: http://www.who.int/immunization/sage/1_WHA56_19_Prevention_and_control_o...
-
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NS, Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR-7):1-60 - PubMed
-
- Grohskopf AE, Sokolow LZ, Olsen SL, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep 2015; 64:818-25; PMID:26247435; http://dx.doi.org/ 10.15585/mmwr.mm6430a3 - DOI - PMC - PubMed
-
- Public Health Agency of Canada Statement on Seasonal Influenza Vaccine for 2014–2015. An Advisory Committee Statement National Advisory Committee on Immunization. Available from: http://www.phac-aspc.gc.ca/naci-ccni/flu-grippe-eng.php
-
- WHO recommendations on the composition of influenza virus vaccines Available from: http://www.who.int/influenza/vaccines/virus/recommendations/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
