Estrogens, Neuroinflammation, and Neurodegeneration
- PMID: 27196727
- PMCID: PMC4971309
- DOI: 10.1210/er.2016-1007
Estrogens, Neuroinflammation, and Neurodegeneration
Abstract
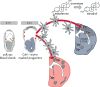
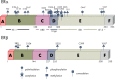
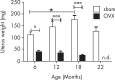
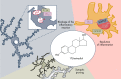
Inflammatory activation of microglia is a hallmark of several disorders of the central nervous system. In addition to protecting the brain against inflammatory insults, microglia are neuroprotective and play a significant role in maintaining neuronal connectivity, but the prolongation of an inflammatory status may limit the beneficial functions of these immune cells. The finding that estrogen receptors are present in monocyte-derived cells and that estrogens prevent and control the inflammatory response raise the question of the role that this sex steroid plays in the manifestation and progression of pathologies that have a clear sex difference in prevalence, such as multiple sclerosis, Parkinson's disease, and Alzheimer's disease. The present review aims to provide a critical review of the current literature on the actions of estrogen in microglia and on the involvement of estrogen receptors in the manifestation of selected neurological disorders. This current understanding highlights a research area that should be expanded to identify appropriate replacement therapies to slow the progression of such diseases.
Figures


References
-
- Friedrich MJ. Research on psychiatric disorders targets inflammation. JAMA. 2014;312(5):474–476. - PubMed
-
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. - PubMed
-
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

