Inhibition of β-Catenin to Overcome Endocrine Resistance in Tamoxifen-Resistant Breast Cancer Cell Line
- PMID: 27196739
- PMCID: PMC4873201
- DOI: 10.1371/journal.pone.0155983
Inhibition of β-Catenin to Overcome Endocrine Resistance in Tamoxifen-Resistant Breast Cancer Cell Line
Abstract
Background: The β-catenin signaling is important in cell growth and differentiation and is frequently dysregulated in various cancers. The most well-known mechanism of endocrine resistance is cross-talk between the estrogen receptor (ER) and other growth factor signaling, such as phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathway. In the present study, we investigated whether β-catenin could be a potential target to overcome endocrine resistance in breast cancer.
Methods: We established tamoxifen-resistant (TamR) cell line via long-term exposure of MCF-7 breast cancer cells to gradually increasing concentrations of tamoxifen. The levels of protein expression and mRNA transcripts were determined using western blot analysis and real-time quantitative PCR. The transcriptional activity of β-catenin was measured using luciferase activity assay.
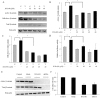
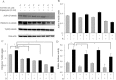
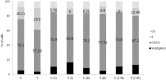
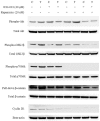
Results: TamR cells showed a mesenchymal phenotype, and exhibited a relatively decreased expression of ER and increased expression of human epidermal growth factor receptor 2 and the epidermal growth factor receptor. We confirmed that the expression and transcriptional activity of β-catenin were increased in TamR cells compared with control cells. The expression and transcriptional activity of β-catenin were inhibited by β-catenin small-molecule inhibitor, ICG-001 or β-catenin siRNA. The viability of TamR cells, which showed no change after treatment with tamoxifen, was reduced by ICG-001 or β-catenin siRNA. The combination of ICG-001 and mTOR inhibitor, rapamycin, yielded an additive effect on the inhibition of viability in TamR cells.
Conclusion: These results suggest that β-catenin plays a role in tamoxifen-resistant breast cancer, and the inhibition of β-catenin may be a potential target in tamoxifen-resistant breast cancer.
Conflict of interest statement
Figures


References
-
- Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

