"Fifty Shades" of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties
- PMID: 27196900
- PMCID: PMC4881568
- DOI: 10.3390/ijms17050746
"Fifty Shades" of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties
Abstract
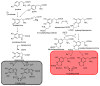
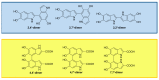
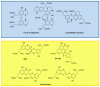
Recent advances in the chemistry of melanins have begun to disclose a number of important structure-property-function relationships of crucial relevance to the biological role of human pigments, including skin (photo) protection and UV-susceptibility. Even slight variations in the monomer composition of black eumelanins and red pheomelanins have been shown to determine significant differences in light absorption, antioxidant, paramagnetic and redox behavior, particle morphology, surface properties, metal chelation and resistance to photo-oxidative wear-and-tear. These variations are primarily governed by the extent of decarboxylation at critical branching points of the eumelanin and pheomelanin pathways, namely the rearrangement of dopachrome to 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA), and the rearrangement of 5-S-cysteinyldopa o-quinoneimine to 1,4-benzothiazine (BTZ) and its 3-carboxylic acid (BTZCA). In eumelanins, the DHICA-to-DHI ratio markedly affects the overall antioxidant and paramagnetic properties of the resulting pigments. In particular, a higher content in DHICA decreases visible light absorption and paramagnetic response relative to DHI-based melanins, but markedly enhances antioxidant properties. In pheomelanins, likewise, BTZCA-related units, prevalently formed in the presence of zinc ions, appear to confer pronounced visible and ultraviolet A (UVA) absorption features, accounting for light-dependent reactive oxygen species (ROS) production, whereas non-carboxylated benzothiazine intermediates seem to be more effective in inducing ROS production by redox cycling mechanisms in the dark. The possible biological and functional significance of carboxyl retention in the eumelanin and pheomelanin pathways is discussed.
Keywords: 5,6-dihydroxyindoles; 5-S-cysteinyldopa; antioxidant; benzothiazines; dopachrome; eumelanin; melanins; melanocortin-1-receptor; pheomelanin; pro-oxidant.
Figures





References
-
- Prota G. The chemistry of melanins and melanogenesis. In: Herz W., Kirby G.W., Moore R.E., Steglich W., Tamm C., editors. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Volume 64. Springer-Verlag; Wien, Austria: 1995. pp. 93–148. - PubMed
-
- Ito S., Wakamatsu K., d’Ischia M., Napolitano A., Pezzella A. Structure of melanins. In: Borovanský J., Riley P.A., editors. Melanins and Melanosomes. Wiley-VCH; Weinheim, Germany: 2011. pp. 167–185.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

