Cell Cycle-Dependent Mechanisms Underlie Vincristine-Induced Death of Primary Acute Lymphoblastic Leukemia Cells
- PMID: 27197148
- PMCID: PMC4911277
- DOI: 10.1158/0008-5472.CAN-15-2104
Cell Cycle-Dependent Mechanisms Underlie Vincristine-Induced Death of Primary Acute Lymphoblastic Leukemia Cells
Abstract
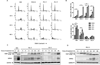
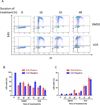
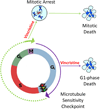
Microtubule-targeting agents (MTA), such as the taxanes and vinca alkaloids, are used to treat a variety of cancers due to their ability to perturb microtubule dynamics. In cell culture, MTAs exert their anticancer effects primarily by causing mitotic arrest and cell death. However, accumulating indirect evidence suggests that MTAs may exert their cytotoxicity in human tumors by interfering with interphase microtubules. In this study, we sought to develop and characterize an experimental system in which to test the hypothesis that MTAs induce cell death during interphase. Primary adult acute lymphoblastic leukemia (ALL) cells treated with vincristine only weakly exhibited colocalization between mitotic and apoptotic markers and major characteristics of mitotic death, such as an increase in cells with 4N DNA content before the appearance of cells with <2N DNA content, suggesting a mixed response. Therefore, we separated ALL cells into distinct phases of the cell cycle by centrifugal elutriation, labeled cells with 5-ethynyl-2'-deoxyuridine (EdU), and then treated each population with vincristine. Cells isolated during G1 underwent cell death without evidence of EdU uptake, indicating that the cytotoxic effects of vincristine took place during G1 Conversely, cells isolated during S or G2-M phases underwent death following mitotic arrest. Thus, vincristine induces distinct death programs in primary ALL cells depending on cell-cycle phase, and cells in G1 are particularly susceptible to perturbation of interphase microtubules. Primary ALL cells may therefore provide a powerful model system in which to study the multimodal mechanisms underlying MTA-induced cell death. Cancer Res; 76(12); 3553-61. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest: No potential conflicts of interest were disclosed.
Figures



Similar articles
-
Primary acute lymphoblastic leukemia cells are susceptible to microtubule depolymerization in G1 and M phases through distinct cell death pathways.J Biol Chem. 2022 Jun;298(6):101939. doi: 10.1016/j.jbc.2022.101939. Epub 2022 Apr 15. J Biol Chem. 2022. PMID: 35436470 Free PMC article.
-
Contrasting effects of microtubule destabilizers versus stabilizers on induction of death in G1 phase of the cell cycle.Biochem Pharmacol. 2019 Apr;162:213-223. doi: 10.1016/j.bcp.2018.12.015. Epub 2018 Dec 19. Biochem Pharmacol. 2019. PMID: 30578765
-
RAD001 (everolimus) induces dose-dependent changes to cell cycle regulation and modifies the cell cycle response to vincristine.Oncogene. 2013 Oct;32(40):4789-97. doi: 10.1038/onc.2012.498. Epub 2012 Nov 5. Oncogene. 2013. PMID: 23128395
-
Exploring the mechanisms of action of the novel microtubule inhibitor vinflunine.Semin Oncol. 2008 Jun;35(3 Suppl 3):S6-S12. doi: 10.1053/j.seminoncol.2008.01.009. Semin Oncol. 2008. PMID: 18538179 Review.
-
Microtubules in apoptosis induction: are they necessary?Curr Cancer Drug Targets. 2007 Dec;7(8):713-29. doi: 10.2174/156800907783220480. Curr Cancer Drug Targets. 2007. PMID: 18220532 Review.
Cited by
-
A Nuclear Long Non-Coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis.Mol Ther. 2021 Jan 6;29(1):263-274. doi: 10.1016/j.ymthe.2020.09.024. Epub 2020 Sep 20. Mol Ther. 2021. PMID: 33002417 Free PMC article.
-
Injectable polypeptide-polysaccharide depot for preventing postoperative tumor recurrence by concurrent in situ chemotherapy and brachytherapy.Mater Today Bio. 2024 Aug 27;28:101219. doi: 10.1016/j.mtbio.2024.101219. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39280112 Free PMC article.
-
Preparation of Primary Acute Lymphoblastic Leukemia Cells in Different Cell Cycle Phases by Centrifugal Elutriation.J Vis Exp. 2017 Nov 10;(129):56418. doi: 10.3791/56418. J Vis Exp. 2017. PMID: 29155772 Free PMC article.
-
Rhopalurus junceus scorpion venom induces G2/M cell cycle arrest and apoptotic cell death in human non-small lung cancer cell lines.J Venom Anim Toxins Incl Trop Dis. 2025 Feb 3;31:e20240035. doi: 10.1590/1678-9199-JVATITD-2024-0035. eCollection 2025. J Venom Anim Toxins Incl Trop Dis. 2025. PMID: 39906356 Free PMC article.
-
Regulatory Mechanisms of the LuxS/AI-2 System and Bacterial Resistance.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01186-19. doi: 10.1128/AAC.01186-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31383657 Free PMC article. Review.
References
-
- Rowinsky EK, Donehower RC. The Chemotherapy Source Book. Baltimore, MD: Williams and Wilkins; 1998. pp. 387–423.
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. - PubMed
-
- Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. - PubMed
-
- Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–2585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

