The mRNA-bound proteome of the early fly embryo
- PMID: 27197210
- PMCID: PMC4937569
- DOI: 10.1101/gr.200386.115
The mRNA-bound proteome of the early fly embryo
Abstract
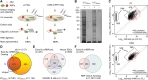
Early embryogenesis is characterized by the maternal to zygotic transition (MZT), in which maternally deposited messenger RNAs are degraded while zygotic transcription begins. Before the MZT, post-transcriptional gene regulation by RNA-binding proteins (RBPs) is the dominant force in embryo patterning. We used two mRNA interactome capture methods to identify RBPs bound to polyadenylated transcripts within the first 2 h of Drosophila melanogaster embryogenesis. We identified a high-confidence set of 476 putative RBPs and confirmed RNA-binding activities for most of 24 tested candidates. Most proteins in the interactome are known RBPs or harbor canonical RBP features, but 99 exhibited previously uncharacterized RNA-binding activity. mRNA-bound RBPs and TFs exhibit distinct expression dynamics, in which the newly identified RBPs dominate the first 2 h of embryonic development. Integrating our resource with in situ hybridization data from existing databases showed that mRNAs encoding RBPs are enriched in posterior regions of the early embryo, suggesting their general importance in posterior patterning and germ cell maturation.
© 2016 Wessels et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Baltz AG, Munschauer M, Schwanhäusser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al. 2012. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell 46: 674–690. - PubMed
-
- Bardsley A, McDonald K, Boswell RE. 1993. Distribution of tudor protein in the Drosophila embryo suggests separation of functions based on site of localization. Development 119: 207–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
