The interaction of PRC2 with RNA or chromatin is mutually antagonistic
- PMID: 27197219
- PMCID: PMC4937559
- DOI: 10.1101/gr.197632.115
The interaction of PRC2 with RNA or chromatin is mutually antagonistic
Abstract
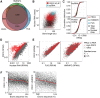
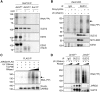
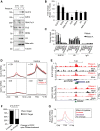
Polycomb repressive complex 2 (PRC2) modifies chromatin to maintain genes in a repressed state during development. PRC2 is primarily associated with CpG islands at repressed genes and also possesses RNA binding activity. However, the RNAs that bind PRC2 in cells, the subunits that mediate these interactions, and the role of RNA in PRC2 recruitment to chromatin all remain unclear. By performing iCLIP for PRC2 in comparison with other RNA binding proteins, we show here that PRC2 binds nascent RNA at essentially all active genes. Although interacting with RNA promiscuously, PRC2 binding is enriched at specific locations within RNAs, primarily exon-intron boundaries and the 3' UTR. Deletion of other PRC2 subunits reveals that SUZ12 is sufficient to establish this RNA binding profile. Contrary to prevailing models, we also demonstrate that the interaction of PRC2 with RNA or chromatin is mutually antagonistic in cells and in vitro. RNA degradation in cells triggers PRC2 recruitment to CpG islands at active genes. Correspondingly, the release of PRC2 from chromatin in cells increases RNA binding. Consistent with this, RNA and nucleosomes compete for PRC2 binding in vitro. We propose that RNA prevents PRC2 recruitment to chromatin at active genes and that mutual antagonism between RNA and chromatin underlies the pattern of PRC2 chromatin association across the genome.
© 2016 Beltran et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Angel A, Song J, Dean C, Howard M. 2011. A Polycomb-based switch underlying quantitative epigenetic memory. Nature 476: 105–108. - PubMed
-
- Bulut-Karslioglu A, De La Rosa-Velazquez IA, Ramirez F, Barenboim M, Onishi-Seebacher M, Arand J, Galan C, Winter GE, Engist B, Gerle B, et al. 2014. Suv39h-dependent H3K9me3 marks intact retrotransposons and silences LINE elements in mouse embryonic stem cells. Mol Cell 55: 277–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
