Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS)
- PMID: 27197775
- PMCID: PMC5113942
- DOI: 10.1016/j.healun.2016.03.005
Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS)
Erratum in
-
Erratum to "Adverse Events in Children Implanted with Ventricular Assist Devices in the US: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PEDIMACS)".J Heart Lung Transplant. 2017 Jan;36(1):116. doi: 10.1016/j.healun.2016.11.003. J Heart Lung Transplant. 2017. PMID: 28109452 No abstract available.
Abstract
Background: Ventricular assist devices (VADs) have been used in children on an increasing basis in recent years. One-year survival rates are now >80% in multiple reports. In this report we describe adverse events experienced by children with durable ventricular assist devices, using a national-level registry (PediMACS, a component of INTERMACS) METHODS: PediMACS is a national registry that contains clinical data on patients who are <19 years of age at the time of VAD implantation. Data collection concludes at the time of VAD explantation. All FDA-approved devices are included. PediMACS was launched on September 1, 2012, and this report includes all data from launch until August 2014. Adverse events were coded with a uniform, pre-specified set of definitions.
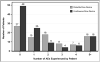
Results: This report comprises data from 200 patients with a median age of 11 years (range 11 days to 18 years), and total follow-up of 783 patient-months. The diagnoses were cardiomyopathy (n = 146, 73%), myocarditis (n = 17, 9%), congenital heart disease (n = 35, 18%) and other (n = 2, 1%). Pulsatile-flow devices were used in 91 patients (45%) and continuous-flow devices in 109 patients (55%). Actuarial survival was 81% at 6 months. There were 418 adverse events reported. The most frequent events were device malfunction (n = 79), infection (n = 78), neurologic dysfunction (n = 52) and bleeding (n = 68). Together, these accounted for 277 events, 66% of the total. Although 38% of patients had no reported adverse event and 16% of patients had ≥5 adverse events. Adverse events occurred at all time-points after implantation, but were most likely to occur in the first 30 days. For continuous-flow devices, there were broad similarities in adverse event rates between this cohort and historic rates from the INTERMACS population.
Conclusions: In this study cohort, the overall rate of early adverse events (within 90 days of implantation) was 86.3 events per 100 patient-months, and of late adverse events it was 20.4 events per 100 patient-months. The most common adverse events in recipients of pulsatile VADs were device malfunction, neurologic dysfunction, bleeding and infection. For continuous-flow VADs, the most common adverse events were infection, bleeding, cardiac arrhythmia, neurologic dysfunction and respiratory failure. Compared with an adult INTERMACS cohort, the overall rate and distribution of adverse events appears similar.
Keywords: heart failure; mechanical circulatory support; pediatrics; pedimacs; registry; ventricular assist device.
Copyright © 2016 International Society for Heart and Lung Transplantation. All rights reserved.
Figures


References
-
- Mansfield RT, Lin KY, Zaoutis T, Mott AR, Mohamad Z, Luan X, Kaufman BD, Ravishankar C, Gaynor JW, Shaddy RE, Rossano JW. The Use of Pediatric Ventricular Assist Devices in Children's Hospitals From 2000 to 2010: Morbidity, Mortality, and Hospital Charges. Pediatr Crit Care Med. 2015 - PubMed
-
- Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113:2313–9. - PubMed
-
- Morales DL, Almond CS, Jaquiss RD, Rosenthal DN, Naftel DC, Massicotte MP, Humpl T, Turrentine MW, Tweddell JS, Cohen GA, Kroslowitz R, Devaney EJ, Canter CE, Fynn-Thompson F, Reinhartz O, Imamura M, Ghanayem NS, Buchholz H, Furness S, Mazor R, Gandhi SK, Fraser CD., Jr Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant. 2011;30:1–8. - PubMed
-
- Fraser CD, Jr, Jaquiss RD, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, Naftel DC, Ichord RN, Bomgaars L, Tweddell JS, Massicotte MP, Turrentine MW, Cohen GA, Devaney EJ, Pearce FB, Carberry KE, Kroslowitz R, Almond CS. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367:532–41. - PubMed
-
- Padalino MA, Bottio T, Tarzia V, Bortolussi G, Cerutti A, Vida VL, Gerosa G, Stellin G. HeartWare ventricular assist device as bridge to transplant in children and adolescents. Artif Organs. 2014;38:418–22. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

