Adding genetic risk score to family history identifies twice as many high-risk men for prostate cancer: Results from the prostate cancer prevention trial
- PMID: 27197965
- PMCID: PMC5501387
- DOI: 10.1002/pros.23200
Adding genetic risk score to family history identifies twice as many high-risk men for prostate cancer: Results from the prostate cancer prevention trial
Abstract
Background: While family history (FH) has been widely used to provide risk information, it captures only a small proportion of subjects with higher genetic susceptibility. Our objective is to assess whether a genetic risk score (GRS) calculated from prostate cancer (PCa) risk-associated single nucleotide polymorphisms (SNPs) can supplement FH for more effective risk stratification for PCa screening decision-making.
Methods: A GRS was calculated based on 29 PCa risk-associated SNPs for 4,528 men of European descent in the placebo arm of the Prostate Cancer Prevention Trial (PCPT). At study entry, participants were free of PCa diagnosis. Performance of FH and GRS were measured by observed detection rate of PCa and high-grade PCa (Gleason score ≥7) during the 7-year study.
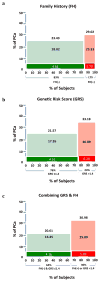
Results: GRS was a significant predictor of PCa in men with or without a positive FH (P = 1.18 × 10(-4) and P = 4.50 × 10(-16) , respectively). Using FH alone, as expected, the 17% of men who were FH+ had a PCa detection rate that was significantly higher (29.02%) than FH- men (23.43%, P = 0.001). When both FH+ or GRS >1.4 are considered, more than twice as many men (36%) can be classified as higher risk, as evidenced by a significantly higher PCa detection rate (30.98%) than in the remaining men (20.61%, P = 5.30 × 10(-15) ). If targeting only FH+ men, four out of five PCa cases would go undetected, as would a similarly large fraction (∼80%) of high-grade PCa cases. In comparison, if targeting FH+ or GRS >1.4 men, almost half of all PCa cases would be detected, including 45% of high-grade PCa cases.
Conclusions: A prostate cancer GRS can supplement family history to better identify higher risk men for targeted intervention. Prostate 76:1120-1129, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: family history; prostate cancer; risk; single nucleotide polymorphisms; the prostate cancer prevention trial.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Jianfeng Xu, Karim Kader, Jielin Sun, S. Lilly Zheng, and William B Isaacs filed several patent applications related to the genetic risk score of prostate cancer risk-associated SNPs.
Figures

References
-
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Paez A, Maattanen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, Kujala PM, Blijenberg BG, Stenman UH, Huber A, Taari K, Hakama M, Moss SM, de Koning HJ, Auvinen A, Investigators E. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. - PMC - PubMed
-
- Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC, Team PP. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132. - PMC - PubMed
-
- Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(11):762–771. - PubMed
-
- Moyer VA Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

