Live single-cell laser tag
- PMID: 27198043
- PMCID: PMC4876456
- DOI: 10.1038/ncomms11636
Live single-cell laser tag
Abstract
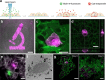
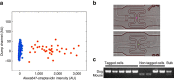
The ability to conduct image-based, non-invasive cell tagging, independent of genetic engineering, is key to cell biology applications. Here we introduce cell labelling via photobleaching (CLaP), a method that enables instant, specific tagging of individual cells based on a wide array of criteria such as shape, behaviour or positional information. CLaP uses laser illumination to crosslink biotin onto the plasma membrane, coupled with streptavidin conjugates to label individual cells for genomic, cell-tracking, flow cytometry or ultra-microscopy applications. We show that the incorporated mark is stable, non-toxic, retained for several days, and transferred by cell division but not to adjacent cells in culture. To demonstrate the potential of CLaP for genomic applications, we combine CLaP with microfluidics-based single-cell capture followed by transcriptome-wide next-generation sequencing. Finally, we show that CLaP can also be exploited for inducing transient cell adhesion to substrates for microengineering cultures with spatially patterned cell types.
Figures



References
-
- Wang M., Orwar O., Olofsson J. & Weber S. G. Single-cell electroporation. Anal. Bioanal. Chem. 397, 3235–3248 (2010). - PubMed
-
- Teissie J., Golzio M. & Rols M. P. Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge. Biochim. Biophys. Acta 1724, 270–280 (2005). - PubMed
-
- Zhang Y. & Yu L. C. Single-cell microinjection technology in cell biology. Bioessays 30, 606–610 (2008). - PubMed
-
- Tsulaia T. V. et al. Glass needle-mediated microinjection of macromolecules and transgenes into primary human mesenchymal stem cells. J. Biomed. Sci. 10, 328–336 (2003). - PubMed
-
- Espina V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

