Nrf2/Keap1 system regulates vascular smooth muscle cell apoptosis for vascular homeostasis: role in neointimal formation after vascular injury
- PMID: 27198574
- PMCID: PMC4873803
- DOI: 10.1038/srep26291
Nrf2/Keap1 system regulates vascular smooth muscle cell apoptosis for vascular homeostasis: role in neointimal formation after vascular injury
Abstract
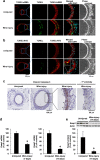
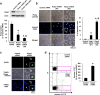
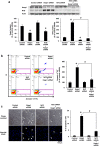
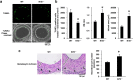
Abnormal increases in vascular smooth muscle cells (VSMCs) in the intimal region after a vascular injury is a key event in developing neointimal hyperplasia. To maintain vascular function, proliferation and apoptosis of VSMCs is tightly controlled during vascular remodeling. NF-E2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) system, a key component of the oxidative stress response that acts in maintaining homeostasis, plays an important role in neointimal hyperplasia after a vascular injury; however, the role of Nrf2/Keap1 in VSMC apoptosis has not been clarified. Here we report that 14 days after arterial injury in mice, TUNEL-positive VSMCs are detected in both the neointimal and medial layers. These layers contain cells expressing high levels of Nrf2 but low Keap1 expression. In VSMCs, Keap1 depletion induces features of apoptosis, such as positive TUNEL staining and annexin V binding. These changes are associated with an increased expression of nuclear Nrf2. Simultaneous Nrf2 depletion inhibits Keap1 depletion-induced apoptosis. At 14 days after the vascular injury, Nrf2-deficient mice demonstrated fewer TUNEL-positive cells and increased neointimal formation in the neointimal and medial areas. The results suggest that the Nrf2/Keap1 system regulates VSMC apoptosis during neointimal formation, thereby inhibiting neointimal hyperplasia after a vascular injury.
Figures



References
-
- Schwartz S. M. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 100, S87–89 (1997). - PubMed
-
- Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond) 114, 257–264 (2008). - PubMed
-
- Slomp J. et al. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arteriosclerosis, thrombosis, and vascular biology 17, 1003–1009 (1997). - PubMed
-
- Durand E. et al. Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol 39, 1680–1685 (2002). - PubMed
-
- Griendling K. K., Sorescu D. & Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86, 494–501 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

