A two-step approach to achieve secondary amide transamidation enabled by nickel catalysis
- PMID: 27199089
- PMCID: PMC4876455
- DOI: 10.1038/ncomms11554
A two-step approach to achieve secondary amide transamidation enabled by nickel catalysis
Abstract
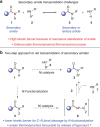
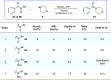
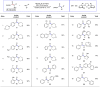
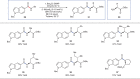
A long-standing challenge in synthetic chemistry is the development of the transamidation reaction. This process, which involves the conversion of one amide to another, is typically plagued by unfavourable kinetic and thermodynamic factors. Although some advances have been made with regard to the transamidation of primary amide substrates, secondary amide transamidation has remained elusive. Here we present a simple two-step approach that allows for the elusive overall transformation to take place using non-precious metal catalysis. The methodology proceeds under exceptionally mild reaction conditions and is tolerant of amino-acid-derived nucleophiles. In addition to overcoming the classic problem of secondary amide transamidation, our studies expand the growing repertoire of new transformations mediated by base metal catalysis.
Figures
Similar articles
-
Nickel-catalyzed transamidation of aliphatic amide derivatives.Chem Sci. 2017 Sep 1;8(9):6433-6438. doi: 10.1039/c7sc01980g. Epub 2017 Jul 10. Chem Sci. 2017. PMID: 29163929 Free PMC article.
-
Conversion of amides to esters by the nickel-catalysed activation of amide C-N bonds.Nature. 2015 Aug 6;524(7563):79-83. doi: 10.1038/nature14615. Epub 2015 Jul 22. Nature. 2015. PMID: 26200342 Free PMC article.
-
Reductive Arylation of Amides via a Nickel-Catalyzed Suzuki-Miyaura-Coupling and Transfer-Hydrogenation Cascade.Angew Chem Int Ed Engl. 2021 Feb 1;60(5):2472-2477. doi: 10.1002/anie.202012048. Epub 2020 Nov 30. Angew Chem Int Ed Engl. 2021. PMID: 33029868 Free PMC article.
-
Direct Transamidation Reactions: Mechanism and Recent Advances.Molecules. 2018 Sep 18;23(9):2382. doi: 10.3390/molecules23092382. Molecules. 2018. PMID: 30231486 Free PMC article. Review.
-
Catalytic synthesis of amides via aldoximes rearrangement.Chem Commun (Camb). 2015 Feb 14;51(13):2495-505. doi: 10.1039/c4cc08684h. Chem Commun (Camb). 2015. PMID: 25503254 Review.
Cited by
-
Benchtop Delivery of Ni(cod)2 using Paraffin Capsules.Org Lett. 2016 Aug 5;18(15):3934-6. doi: 10.1021/acs.orglett.6b01758. Epub 2016 Jul 25. Org Lett. 2016. PMID: 27454146 Free PMC article.
-
Nickel-Catalyzed Suzuki-Miyaura Coupling of Aliphatic Amides.ACS Catal. 2018 Feb 2;8(2):1003-1008. doi: 10.1021/acscatal.7b03688. Epub 2017 Dec 20. ACS Catal. 2018. PMID: 29682398 Free PMC article.
-
Transamidation of Amides and Amidation of Esters by Selective N-C(O)/O-C(O) Cleavage Mediated by Air- and Moisture-Stable Half-Sandwich Nickel(II)-NHC Complexes.Molecules. 2021 Jan 2;26(1):188. doi: 10.3390/molecules26010188. Molecules. 2021. PMID: 33401664 Free PMC article.
-
Enantioselective nickel-catalyzed Mizoroki-Heck cyclizations of amide electrophiles.Chem Sci. 2024 Jan 8;15(7):2593-2600. doi: 10.1039/d3sc05797f. eCollection 2024 Feb 14. Chem Sci. 2024. PMID: 38362425 Free PMC article.
-
Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins.Nat Commun. 2017 May 5;8:14993. doi: 10.1038/ncomms14993. Nat Commun. 2017. PMID: 28474671 Free PMC article.
References
-
- Corey E. J. & Cheng X.-M. The Logic of Chemical Synthesis Wiley-VCH (1995).
-
- Hudlicky T. & Reed J. W. The Way of Synthesis: Evolution of Design and Methods for Natural Products Wiley-VCH (2007).
-
- González-Rosende M. E., Castillo E., Lasri J. & Sepúlveda-Arques J. Intermolecular and intramolecular transamidation reactions. Prog. React. Kinet. Mech. 29, 311 (2004).
-
- Pattabiraman V. R. & Bode J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011). - PubMed
-
- Loomis W. D. & Stumpf P. K. in Transamination and Transamidation (Nitrogen Metabolism) eds Allen E. K.et al.. 249–250Springer Berlin Heidelberg (1958).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

