Disrupted NOS signaling in lymphatic endothelial cells exposed to chronically increased pulmonary lymph flow
- PMID: 27199125
- PMCID: PMC4967199
- DOI: 10.1152/ajpheart.00649.2015
Disrupted NOS signaling in lymphatic endothelial cells exposed to chronically increased pulmonary lymph flow
Abstract
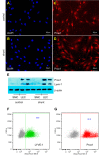
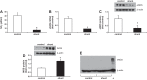
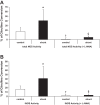
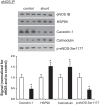
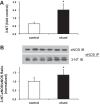
Associated abnormalities of the lymphatic circulation are well described in congenital heart disease. However, their mechanisms remain poorly elucidated. Using a clinically relevant ovine model of a congenital cardiac defect with chronically increased pulmonary blood flow (shunt), we previously demonstrated that exposure to chronically elevated pulmonary lymph flow is associated with: 1) decreased bioavailable nitric oxide (NO) in pulmonary lymph; and 2) attenuated endothelium-dependent relaxation of thoracic duct rings, suggesting disrupted lymphatic endothelial NO signaling in shunt lambs. To further elucidate the mechanisms responsible for this altered NO signaling, primary lymphatic endothelial cells (LECs) were isolated from the efferent lymphatic of the caudal mediastinal node in 4-wk-old control and shunt lambs. We found that shunt LECs (n = 3) had decreased bioavailable NO and decreased endothelial nitric oxide synthase (eNOS) mRNA and protein expression compared with control LECs (n = 3). eNOS activity was also low in shunt LECs, but, interestingly, inducible nitric oxide synthase (iNOS) expression and activity were increased in shunt LECs, as were total cellular nitration, including eNOS-specific nitration, and accumulation of reactive oxygen species (ROS). Pharmacological inhibition of iNOS reduced ROS in shunt LECs to levels measured in control LECs. These data support the conclusion that NOS signaling is disrupted in the lymphatic endothelium of lambs exposed to chronically increased pulmonary blood and lymph flow and may contribute to decreased pulmonary lymphatic bioavailable NO.
Keywords: nitric oxide signaling; nitric oxide synthase.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
KLF2-mediated disruption of PPAR-γ signaling in lymphatic endothelial cells exposed to chronically increased pulmonary lymph flow.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H173-H181. doi: 10.1152/ajpheart.00635.2017. Epub 2018 Apr 6. Am J Physiol Heart Circ Physiol. 2018. PMID: 29631374 Free PMC article.
-
Altered reactivity and nitric oxide signaling in the isolated thoracic duct from an ovine model of congenital heart disease with increased pulmonary blood flow.Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H954-62. doi: 10.1152/ajpheart.00841.2013. Epub 2014 Feb 14. Am J Physiol Heart Circ Physiol. 2014. PMID: 24531811 Free PMC article.
-
HIF-1α promotes cellular growth in lymphatic endothelial cells exposed to chronically elevated pulmonary lymph flow.Sci Rep. 2021 Jan 14;11(1):1468. doi: 10.1038/s41598-020-80882-1. Sci Rep. 2021. PMID: 33446832 Free PMC article.
-
Inducible nitric oxide synthase: Regulation, structure, and inhibition.Med Res Rev. 2020 Jan;40(1):158-189. doi: 10.1002/med.21599. Epub 2019 Jun 13. Med Res Rev. 2020. PMID: 31192483 Free PMC article. Review.
-
Therapeutic Potentials of Near-Infrared II Photobiomodulation to Treat Cerebrovascular Diseases via Nitric Oxide Signalling.Adv Exp Med Biol. 2024;1463:195-200. doi: 10.1007/978-3-031-67458-7_33. Adv Exp Med Biol. 2024. PMID: 39400823 Free PMC article. Review.
Cited by
-
Kidney injury-mediated disruption of intestinal lymphatics involves dicarbonyl-modified lipoproteins.Kidney Int. 2021 Sep;100(3):585-596. doi: 10.1016/j.kint.2021.05.028. Epub 2021 Jun 5. Kidney Int. 2021. PMID: 34102217 Free PMC article.
-
Effect of Human Synovial Fluid From Osteoarthritis Patients and Healthy Individuals on Lymphatic Contractile Activity.J Biomech Eng. 2022 Jul 1;144(7):071012. doi: 10.1115/1.4053749. J Biomech Eng. 2022. PMID: 35118490 Free PMC article.
-
Lymphatic Vessel Network Structure and Physiology.Compr Physiol. 2018 Dec 13;9(1):207-299. doi: 10.1002/cphy.c180015. Compr Physiol. 2018. PMID: 30549020 Free PMC article. Review.
-
Lymphatic Vessels and Their Surroundings: How Local Physical Factors Affect Lymph Flow.Biology (Basel). 2020 Dec 11;9(12):463. doi: 10.3390/biology9120463. Biology (Basel). 2020. PMID: 33322476 Free PMC article. Review.
-
Apelin-VEGF-C mRNA delivery as therapeutic for the treatment of secondary lymphedema.EMBO Mol Med. 2024 Feb;16(2):386-415. doi: 10.1038/s44321-023-00017-7. Epub 2024 Jan 2. EMBO Mol Med. 2024. PMID: 38177539 Free PMC article.
References
-
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci 75: 639–653, 2004. - PubMed
-
- Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res 43: 521–531, 1999. - PubMed
-
- Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009. - PubMed
-
- Black SM, Bekker JM, Johengen MJ, Parry AJ, Soifer SJ, Fineman JR. Altered regulation of the ET-1 cascade in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res 47: 97–106, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

