Potential Involvement of Draxin in the Axonal Projection of Cranial Nerves, Especially Cranial Nerve X, in the Chick Hindbrain
- PMID: 27199282
- PMCID: PMC4931760
- DOI: 10.1369/0022155416646538
Potential Involvement of Draxin in the Axonal Projection of Cranial Nerves, Especially Cranial Nerve X, in the Chick Hindbrain
Abstract
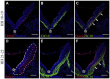
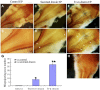
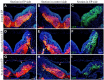
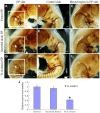
The appropriate projection of axons within the nervous system is a crucial component of the establishment of neural circuitry. Draxin is a repulsive axon guidance protein. Draxin has important functions in the guidance of three commissures in the central nervous system and in the migration of neural crest cells and dI3 interneurons in the chick spinal cord. Here, we report that the distribution of the draxin protein and the location of 23C10-positive areas have a strong temporal and spatial correlation. The overexpression of draxin, especially transmembrane draxin, caused 23C10-positive axon bundles to misproject in the dorsal hindbrain. In addition, the overexpression of transmembrane draxin caused abnormal formation of the ganglion crest of the IX and X cranial nerves, misprojection of some anti-human natural killer-1 (HNK-1)-stained structures in the dorsal roof of the hindbrain, and a simultaneous reduction in the efferent nerves of some motoneuron axons inside the hindbrain. Our data reveal that draxin might be involved in the fascicular projection of cranial nerves in the hindbrain.
Keywords: axon projection; chick; cranial nerve; draxin; hindbrain.
© 2016 The Histochemical Society.
Conflict of interest statement
Figures

Similar articles
-
Draxin, an axon guidance protein, affects chick trunk neural crest migration.Dev Growth Differ. 2009 Dec;51(9):787-96. doi: 10.1111/j.1440-169X.2009.01137.x. Epub 2009 Oct 12. Dev Growth Differ. 2009. PMID: 19824897
-
Rhombomere origin plays a role in the specificity of cranial motor axon projections in the chick.Eur J Neurosci. 1999 Apr;11(4):1403-13. doi: 10.1046/j.1460-9568.1999.00559.x. Eur J Neurosci. 1999. PMID: 10103135
-
A potential inhibitory function of draxin in regulating mouse trunk neural crest migration.In Vitro Cell Dev Biol Anim. 2017 Jan;53(1):43-53. doi: 10.1007/s11626-016-0079-0. Epub 2016 Sep 20. In Vitro Cell Dev Biol Anim. 2017. PMID: 27649978 Free PMC article.
-
[Functional anatomy of the glossopharyngeal, vagus, accessory and hypoglossal cranial nerves].Neurochirurgie. 2009 Apr;55(2):132-5. doi: 10.1016/j.neuchi.2009.01.018. Epub 2009 Mar 21. Neurochirurgie. 2009. PMID: 19304301 Review. French.
-
Evolutionary patterns of cranial nerve efferent nuclei in vertebrates.Brain Behav Evol. 2005;66(4):234-54. doi: 10.1159/000088128. Epub 2005 Oct 25. Brain Behav Evol. 2005. PMID: 16254413 Review.
Cited by
-
Olanzapine Increases Neural Chemorepulsant-Draxin Expression in the Adult Rat Hippocampus.Pharmaceuticals (Basel). 2021 Mar 27;14(4):298. doi: 10.3390/ph14040298. Pharmaceuticals (Basel). 2021. PMID: 33801609 Free PMC article.
References
-
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990;13:329–35. - PubMed
-
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–15. - PubMed
-
- Culotti JG, Kolodkin AL. Functions of netrins and semaphorins in axon guidance. Curr Opin Neurobiol. 1996;6:81–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

