Meiotic onset is reliant on spatial distribution but independent of germ cell number in the mouse ovary
- PMID: 27199373
- PMCID: PMC5930833
- DOI: 10.1242/jcs.189910
Meiotic onset is reliant on spatial distribution but independent of germ cell number in the mouse ovary
Abstract
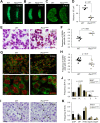
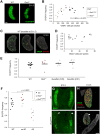
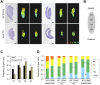
Mouse ovarian germ cells enter meiosis in a wave that propagates from anterior to posterior, but little is known about contribution of germ cells to initiation or propagation of meiosis. In a Ror2 mutant with diminished germ cell number and migration, we find that overall timing of meiotic initiation is delayed at the population level. We use chemotherapeutic depletion to exclude a profoundly reduced number of germ cells as a cause for meiotic delay. We rule out sex reversal or failure to specify somatic support cells as contributors to the meiotic phenotype. Instead, we find that anomalies in the distribution of germ cells as well as gonad shape in mutants contribute to aberrant initiation of meiosis. Our analysis supports a model of meiotic initiation via diffusible signal(s), excludes a role for germ cells in commencing the meiotic wave and furnishes the first phenotypic demonstration of the wave of meiotic entry. Finally, our studies underscore the importance of considering germ cell migration defects while studying meiosis to discern secondary effects resulting from positioning versus primary meiotic entry phenotypes.
Keywords: Germ cell; Gonad; Meiosis; Migration; Ror2; Wave.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
-
- Abby E., Tourpin S., Ribeiro J., Daniel K., Messiaen S., Moison D., Guerquin J., Gaillard J-C, Armengaud J., Langa F. et al. (2016). Implementation of meiosis prophase I programme requires a conserved retinoid-independent stabilizer of meiotic transcripts. Nat. Commun. 7, 10324 10.1038/ncomms10324 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

