Effect of selective expression of dominant-negative PPARγ in pro-opiomelanocortin neurons on the control of energy balance
- PMID: 27199455
- PMCID: PMC4967222
- DOI: 10.1152/physiolgenomics.00032.2016
Effect of selective expression of dominant-negative PPARγ in pro-opiomelanocortin neurons on the control of energy balance
Abstract
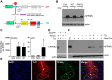
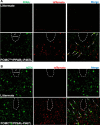
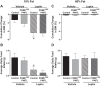
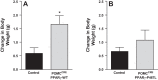
Peroxisome proliferator-activated receptor-γ (PPARγ), a master regulator of adipogenesis, was recently shown to affect energy homeostasis through its actions in the brain. Deletion of PPARγ in mouse brain, and specifically in the pro-opiomelanocortin (POMC) neurons, results in resistance to diet-induced obesity. To study the mechanisms by which PPARγ in POMC neurons controls energy balance, we constructed a Cre-recombinase-dependent conditionally activatable transgene expressing either wild-type (WT) or dominant-negative (P467L) PPARγ and the tdTomato reporter. Inducible expression of both forms of PPARγ was validated in cells in culture, in liver of mice infected with an adenovirus expressing Cre-recombinase (AdCre), and in the brain of mice expressing Cre-recombinase either in all neurons (NES(Cre)/PPARγ-P467L) or selectively in POMC neurons (POMC(Cre)/PPARγ-P467L). Whereas POMC(Cre)/PPARγ-P467L mice exhibited a normal pattern of weight gain when fed 60% high-fat diet, they exhibited increased weight gain and fat mass accumulation in response to a 10% fat isocaloric-matched control diet. POMC(Cre)/PPARγ-P467L mice were leptin sensitive on control diet but became leptin resistant when fed 60% high-fat diet. There was no difference in body weight between POMC(Cre)/PPARγ-WT mice and controls in response to 60% high-fat diet. However, POMC(Cre)/PPARγ-WT, but not POMC(Cre)/PPARγ-P467L, mice increased body weight in response to rosiglitazone, a PPARγ agonist. These observations support the concept that alterations in PPARγ-driven mechanisms in POMC neurons can play a role in the regulation of metabolic homeostasis under certain dietary conditions.
Keywords: POMC; PPARγ; neuron; rosiglitazone.
Copyright © 2016 the American Physiological Society.
Figures


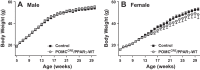
References
-
- Balsevich G, Uribe A, Wagner KV, Hartmann J, Santarelli S, Labermaier C, Schmidt MV. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J Endocrinol 222: 15–26, 2014. - PubMed
-
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402: 880–883, 1999. - PubMed
-
- Burke LK, Doslikova B, D'Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, Garfield AS, Apergis-Schoute J, Lam DD, Speakman JR, Rubinstein M, Low MJ, Rochford JJ, Myers MG, Evans ML, Heisler LK. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab 5: 245–252, 2016. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

