Retinoic acid and sodium butyrate suppress the cardiac expression of hypertrophic markers and proinflammatory mediators in Npr1 gene-disrupted haplotype mice
- PMID: 27199456
- PMCID: PMC4967220
- DOI: 10.1152/physiolgenomics.00073.2015
Retinoic acid and sodium butyrate suppress the cardiac expression of hypertrophic markers and proinflammatory mediators in Npr1 gene-disrupted haplotype mice
Abstract
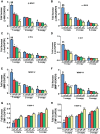
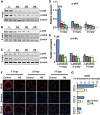
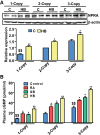
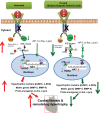
The objective of the present study was to examine the genetically determined differences in the natriuretic peptide receptor-A (NPRA) gene (Npr1) copies affecting the expression of cardiac hypertrophic markers, proinflammatory mediators, and matrix metalloproteinases (MMPs) in a gene-dose-dependent manner. We determined whether stimulation of Npr1 by all-trans retinoic acid (RA) and histone deacetylase (HDAC) inhibitor sodium butyric acid (SB) suppress the expression of cardiac disease markers. In the present study, we utilized Npr1 gene-disrupted heterozygous (Npr1(+/-), 1-copy), wild-type (Npr1(+/+), 2-copy), gene-duplicated (Npr1(++/+), 3-copy) mice, which were treated intraperitoneally with RA, SB, and a combination of RA/SB, a hybrid drug (HB) for 2 wk. Untreated 1-copy mice showed significantly increased heart weight-body weight (HW/BW) ratio, blood pressure, hypertrophic markers, including beta-myosin heavy chain (β-MHC) and proto-oncogenes (c-fos and c-jun), proinflammatory mediator nuclear factor kappa B (NF-κB), and MMPs (MMP-2, MMP-9) compared with 2-copy and 3-copy mice. The heterozygous (haplotype) 1-copy mice treated with RA, SB, or HB, exhibited significant reduction in the expression of β-MHC, c-fos, c-jun, NF-κB, MMP-2, and MMP-9. In drug-treated animals, the activity and expression levels of HDAC were significantly reduced and histone acetyltransferase activity and expression levels were increased. The drug treatments significantly increased the fractional shortening and reduced the systolic and diastolic parameters of the Npr1(+/-) mice hearts. Together, the present results demonstrate that a decreased Npr1 copy number enhanced the expression of hypertrophic markers, proinflammatory mediators, and MMPs, whereas an increased Npr1 repressed the cardiac disease markers in a gene-dose-dependent manner.
Keywords: fibrosis; gene-disruption; histone acetyltransferase; histone deacetylase; hypertrophy; natriuretic peptide receptor-A.
Copyright © 2016 the American Physiological Society.
Figures




Similar articles
-
Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F781-F795. doi: 10.1152/ajprenal.00166.2017. Epub 2017 May 31. Am J Physiol Renal Physiol. 2017. PMID: 28566502 Free PMC article.
-
Genetically altered mutant mouse models of guanylyl cyclase/natriuretic peptide receptor-A exhibit the cardiac expression of proinflammatory mediators in a gene-dose-dependent manner.Endocrinology. 2014 Mar;155(3):1045-56. doi: 10.1210/en.2013-1416. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424043 Free PMC article.
-
All-trans retinoic acid and sodium butyrate enhance natriuretic peptide receptor a gene transcription: role of histone modification.Mol Pharmacol. 2014 Jun;85(6):946-57. doi: 10.1124/mol.114.092221. Epub 2014 Apr 8. Mol Pharmacol. 2014. PMID: 24714214 Free PMC article.
-
Regulation of cardiac angiotensin-converting enzyme and angiotensin AT1 receptor gene expression in Npr1 gene-disrupted mice.Clin Exp Pharmacol Physiol. 2010 Feb;37(2):e70-7. doi: 10.1111/j.1440-1681.2009.05315.x. Epub 2009 Oct 16. Clin Exp Pharmacol Physiol. 2010. PMID: 19843097 Free PMC article. Review.
-
Molecular and genetic aspects of guanylyl cyclase natriuretic peptide receptor-A in regulation of blood pressure and renal function.Physiol Genomics. 2018 Nov 1;50(11):913-928. doi: 10.1152/physiolgenomics.00083.2018. Epub 2018 Aug 31. Physiol Genomics. 2018. PMID: 30169131 Free PMC article. Review.
Cited by
-
Guanylyl cyclase/natriuretic peptide receptor-A: Identification, molecular characterization, and physiological genomics.Front Mol Neurosci. 2023 Jan 4;15:1076799. doi: 10.3389/fnmol.2022.1076799. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36683859 Free PMC article. Review.
-
Genetic Disruption of Guanylyl Cyclase/Natriuretic Peptide Receptor-A Triggers Differential Cardiac Fibrosis and Disorders in Male and Female Mutant Mice: Role of TGF-β1/SMAD Signaling Pathway.Int J Mol Sci. 2022 Sep 29;23(19):11487. doi: 10.3390/ijms231911487. Int J Mol Sci. 2022. PMID: 36232788 Free PMC article.
-
Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas.Cell Rep. 2018 Apr 3;23(1):194-212.e6. doi: 10.1016/j.celrep.2018.03.063. Cell Rep. 2018. PMID: 29617660 Free PMC article.
-
Molecular Signaling Mechanisms and Function of Natriuretic Peptide Receptor-A in the Pathophysiology of Cardiovascular Homeostasis.Front Physiol. 2021 Aug 19;12:693099. doi: 10.3389/fphys.2021.693099. eCollection 2021. Front Physiol. 2021. PMID: 34489721 Free PMC article. Review.
-
Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease.Front Cardiovasc Med. 2022 Aug 11;9:900381. doi: 10.3389/fcvm.2022.900381. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035928 Free PMC article. Review.
References
-
- Arise KK, Pandey KN. Inhibition and down-regulation of gene transcription and guanylyl cyclase activity of NPRA by angiotensin II involving protein kinase C. Biochem Biophys Res Commun 349: 131–135, 2006. - PubMed
-
- Austenaa LM, Carlsen H, Ertesvag A, Alexander G, Blomhoff HK, Blomhoff R. Vitamin A status significantly alters nuclear factor-kappaB activity assessed by in vivo imaging. FASEB J 18: 1255–1257, 2004. - PubMed
-
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nature Struct Mol Biol 14: 1008–1016, 2007. - PubMed
-
- Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res 106: 272–284, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

