More Gamma More Predictions: Gamma-Synchronization as a Key Mechanism for Efficient Integration of Classical Receptive Field Inputs with Surround Predictions
- PMID: 27199684
- PMCID: PMC4842768
- DOI: 10.3389/fnsys.2016.00035
More Gamma More Predictions: Gamma-Synchronization as a Key Mechanism for Efficient Integration of Classical Receptive Field Inputs with Surround Predictions
Abstract
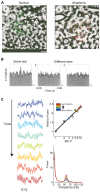
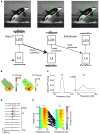
During visual stimulation, neurons in visual cortex often exhibit rhythmic and synchronous firing in the gamma-frequency (30-90 Hz) band. Whether this phenomenon plays a functional role during visual processing is not fully clear and remains heavily debated. In this article, we explore the function of gamma-synchronization in the context of predictive and efficient coding theories. These theories hold that sensory neurons utilize the statistical regularities in the natural world in order to improve the efficiency of the neural code, and to optimize the inference of the stimulus causes of the sensory data. In visual cortex, this relies on the integration of classical receptive field (CRF) data with predictions from the surround. Here we outline two main hypotheses about gamma-synchronization in visual cortex. First, we hypothesize that the precision of gamma-synchronization reflects the extent to which CRF data can be accurately predicted by the surround. Second, we hypothesize that different cortical columns synchronize to the extent that they accurately predict each other's CRF visual input. We argue that these two hypotheses can account for a large number of empirical observations made on the stimulus dependencies of gamma-synchronization. Furthermore, we show that they are consistent with the known laminar dependencies of gamma-synchronization and the spatial profile of intercolumnar gamma-synchronization, as well as the dependence of gamma-synchronization on experience and development. Based on our two main hypotheses, we outline two additional hypotheses. First, we hypothesize that the precision of gamma-synchronization shows, in general, a negative dependence on RF size. In support, we review evidence showing that gamma-synchronization decreases in strength along the visual hierarchy, and tends to be more prominent in species with small V1 RFs. Second, we hypothesize that gamma-synchronized network dynamics facilitate the emergence of spiking output that is particularly information-rich and sparse.
Keywords: V1; communication through coherence; efficient coding; gamma oscilations; gamma synchrony; laminar organization; predictive coding; surround suppression.
Figures



Similar articles
-
Surface color and predictability determine contextual modulation of V1 firing and gamma oscillations.Elife. 2019 Feb 4;8:e42101. doi: 10.7554/eLife.42101. Elife. 2019. PMID: 30714900 Free PMC article.
-
Layer 3 Dynamically Coordinates Columnar Activity According to Spatial Context.J Neurosci. 2019 Jan 9;39(2):281-294. doi: 10.1523/JNEUROSCI.1568-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459226 Free PMC article.
-
Predictive coding of natural images by V1 firing rates and rhythmic synchronization.Neuron. 2022 Apr 6;110(7):1240-1257.e8. doi: 10.1016/j.neuron.2022.01.002. Epub 2022 Feb 3. Neuron. 2022. PMID: 35120628 Free PMC article.
-
Surround suppression supports second-order feature encoding by macaque V1 and V2 neurons.Vision Res. 2014 Nov;104:24-35. doi: 10.1016/j.visres.2014.10.004. Epub 2014 Oct 23. Vision Res. 2014. PMID: 25449336 Free PMC article. Review.
-
Neuronal coherence during selective attentional processing and sensory-motor integration.J Physiol Paris. 2006 Oct;100(4):182-93. doi: 10.1016/j.jphysparis.2007.01.005. Epub 2007 Jan 17. J Physiol Paris. 2006. PMID: 17317118 Review.
Cited by
-
Dynamic fading memory and expectancy effects in the monkey primary visual cortex.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314855121. doi: 10.1073/pnas.2314855121. Epub 2024 Feb 14. Proc Natl Acad Sci U S A. 2024. PMID: 38354261 Free PMC article.
-
An image-computable model for the stimulus selectivity of gamma oscillations.Elife. 2019 Nov 8;8:e47035. doi: 10.7554/eLife.47035. Elife. 2019. PMID: 31702552 Free PMC article.
-
Functionally Distinct Gamma Range Activity Revealed by Stimulus Tuning in Human Visual Cortex.Curr Biol. 2019 Oct 21;29(20):3345-3358.e7. doi: 10.1016/j.cub.2019.08.004. Epub 2019 Oct 3. Curr Biol. 2019. PMID: 31588003 Free PMC article.
-
Classic Psychedelic Drugs: Update on Biological Mechanisms.Pharmacopsychiatry. 2022 May;55(3):121-138. doi: 10.1055/a-1721-2914. Epub 2022 Jan 25. Pharmacopsychiatry. 2022. PMID: 35079988 Free PMC article.
-
Synaptic plasticity facilitates oscillations in a V1 cortical column model with multiple interneuron types.Front Comput Neurosci. 2025 Apr 30;19:1568143. doi: 10.3389/fncom.2025.1568143. eCollection 2025. Front Comput Neurosci. 2025. PMID: 40370493 Free PMC article.
References
-
- Abeles M. (1982). Role of the cortical neuron: integrator or coincidence detector? Isr. J. Med. Sci. 18, 83–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

