Traumatic Brain Injury Alters Methionine Metabolism: Implications for Pathophysiology
- PMID: 27199685
- PMCID: PMC4850826
- DOI: 10.3389/fnsys.2016.00036
Traumatic Brain Injury Alters Methionine Metabolism: Implications for Pathophysiology
Abstract
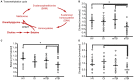
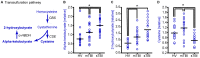
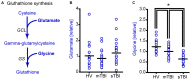
Methionine is an essential proteinogenic amino acid that is obtained from the diet. In addition to its requirement for protein biosynthesis, methionine is metabolized to generate metabolites that play key roles in a number of cellular functions. Metabolism of methionine via the transmethylation pathway generates S-adenosylmethionine (SAM) that serves as the principal methyl (-CH3) donor for DNA and histone methyltransferases (MTs) to regulate epigenetic changes in gene expression. SAM is also required for methylation of other cellular proteins that serve various functions and phosphatidylcholine synthesis that participate in cellular signaling. Under conditions of oxidative stress, homocysteine (which is derived from SAM) enters the transsulfuration pathway to generate glutathione, an important cytoprotective molecule against oxidative damage. As both experimental and clinical studies have shown that traumatic brain injury (TBI) alters DNA and histone methylation and causes oxidative stress, we examined if TBI alters the plasma levels of methionine and its metabolites in human patients. Blood samples were collected from healthy volunteers (HV; n = 20) and patients with mild TBI (mTBI; GCS > 12; n = 20) or severe TBI (sTBI; GCS < 8; n = 20) within the first 24 h of injury. The levels of methionine and its metabolites in the plasma samples were analyzed by either liquid chromatography-mass spectrometry or gas chromatography-mass spectrometry (LC-MS or GC-MS). sTBI decreased the levels of methionine, SAM, betaine and 2-methylglycine as compared to HV, indicating a decrease in metabolism through the transmethylation cycle. In addition, precursors for the generation of glutathione, cysteine and glycine were also found to be decreased as were intermediate metabolites of the gamma-glutamyl cycle (gamma-glutamyl amino acids and 5-oxoproline). mTBI also decreased the levels of methionine, α-ketobutyrate, 2 hydroxybutyrate and glycine, albeit to lesser degrees than detected in the sTBI group. Taken together, these results suggest that decreased levels of methionine and its metabolic products are likely to alter cellular function in multiple organs at a systems level.
Keywords: S-adenosylmethionine; concussion; epigenetic changes; metabolomics; protein methylation; transsulfuration.
Figures



Similar articles
-
A quantitative metabolomics assay targeting 14 intracellular metabolites associated with the methionine transsulfuration pathway using LC-MS/MS in breast cancer cells.J Chromatogr B Analyt Technol Biomed Life Sci. 2022 Aug 1;1205:123314. doi: 10.1016/j.jchromb.2022.123314. Epub 2022 Jun 2. J Chromatogr B Analyt Technol Biomed Life Sci. 2022. PMID: 35772357
-
Metabolic changes associated with methionine stress sensitivity in MDA-MB-468 breast cancer cells.Cancer Metab. 2016 May 2;4:9. doi: 10.1186/s40170-016-0148-6. eCollection 2016. Cancer Metab. 2016. PMID: 27141305 Free PMC article.
-
Oxidative stress-induced regulation of the methionine metabolic pathway in human lung epithelial-like (A549) cells.Mutat Res. 2009 Mar 31;674(1-2):23-30. doi: 10.1016/j.mrgentox.2008.10.006. Epub 2008 Oct 25. Mutat Res. 2009. PMID: 19010443
-
Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine.Clin Chem Lab Med. 2007;45(12):1694-9. doi: 10.1515/CCLM.2007.341. Clin Chem Lab Med. 2007. PMID: 17963455 Review.
-
B-Vitamin dependent methionine metabolism and alcoholic liver disease.Clin Chem Lab Med. 2013 Mar 1;51(3):457-65. doi: 10.1515/cclm-2012-0308. Clin Chem Lab Med. 2013. PMID: 23096111 Review.
Cited by
-
How tissue damage MET metabolism: Regulation of the systemic damage response.Fly (Austin). 2017 Jan 2;11(1):27-36. doi: 10.1080/19336934.2016.1221549. Epub 2016 Aug 11. Fly (Austin). 2017. PMID: 27562340 Free PMC article. Review.
-
Urinary metabolites predict mortality or need for renal replacement therapy after combat injury.Crit Care. 2021 Mar 23;25(1):119. doi: 10.1186/s13054-021-03544-2. Crit Care. 2021. PMID: 33757577 Free PMC article.
-
Differential expression of m5C RNA methyltransferase genes NSUN6 and NSUN7 in Alzheimer's disease and traumatic brain injury.Mol Neurobiol. 2023 Apr;60(4):2223-2235. doi: 10.1007/s12035-022-03195-6. Epub 2023 Jan 17. Mol Neurobiol. 2023. PMID: 36646969 Free PMC article.
-
Serum Levels of Lipids and Selected Aminothiols in Epileptic Children-A Pilot Case-Control Study.Brain Sci. 2022 Jan 17;12(1):120. doi: 10.3390/brainsci12010120. Brain Sci. 2022. PMID: 35053863 Free PMC article.
-
Neuroprotection or Sex Bias: A Protective Response to Traumatic Brain Injury in the Females.CNS Neurol Disord Drug Targets. 2024;23(7):906-916. doi: 10.2174/1871527323666230817102125. CNS Neurol Disord Drug Targets. 2024. PMID: 37592792 Review.
References
-
- Bayir H., Kagan V. E., Tyurina Y. Y., Tyurin V., Ruppel R. A., Adelson P. D., et al. . (2002). Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 51, 571–578. 10.1203/00006450-200205000-00005 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

