Enteral Glutamine Administration in Critically Ill Nonseptic Patients Does Not Trigger Arginine Synthesis
- PMID: 27200186
- PMCID: PMC4855021
- DOI: 10.1155/2016/1373060
Enteral Glutamine Administration in Critically Ill Nonseptic Patients Does Not Trigger Arginine Synthesis
Abstract
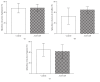
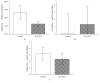
Glutamine supplementation in specific groups of critically ill patients results in favourable clinical outcome. Enhancement of citrulline and arginine synthesis by glutamine could serve as a potential mechanism. However, while receiving optimal enteral nutrition, uptake and enteral metabolism of glutamine in critically ill patients remain unknown. Therefore we investigated the effect of a therapeutically relevant dose of L-glutamine on synthesis of L-citrulline and subsequent L-arginine in this group. Ten versus ten critically ill patients receiving full enteral nutrition, or isocaloric isonitrogenous enteral nutrition including 0.5 g/kg L-alanyl-L-glutamine, were studied using stable isotopes. A cross-over design using intravenous and enteral tracers enabled splanchnic extraction (SE) calculations. Endogenous rate of appearance and SE of glutamine citrulline and arginine was not different (SE controls versus alanyl-glutamine: glutamine 48 and 48%, citrulline 33 versus 45%, and arginine 45 versus 42%). Turnover from glutamine to citrulline and arginine was not higher in glutamine-administered patients. In critically ill nonseptic patients receiving adequate nutrition and a relevant dose of glutamine there was no extra citrulline or arginine synthesis and glutamine SE was not increased. This suggests that for arginine synthesis enhancement there is no need for an additional dose of glutamine when this population is adequately fed. This trial is registered with NTR2285.
Figures

References
-
- Déchelotte P., Hasselmann M., Cynober L., et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Critical Care Medicine. 2006;34(3):598–604. doi: 10.1097/01.ccm.0000201004.30750.d1. - DOI - PubMed
-
- Jian Z. M., Cao J. D., Zhu X. G., et al. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double-blind, controlled study of 120 patients. Journal of Parenteral and Enteral Nutrition. 1999;23(5):S62–S66. doi: 10.1177/014860719902300516. - DOI - PubMed
-
- Scheppach W., Loges C., Bartram P., et al. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107(2):429–434. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

