Obesity but not high-fat diet impairs lymphatic function
- PMID: 27200507
- PMCID: PMC5050064
- DOI: 10.1038/ijo.2016.96
Obesity but not high-fat diet impairs lymphatic function
Abstract
Background/objectives: High-fat diet (HFD)-induced obesity has significant negative effects on lymphatic function, but it remains unclear whether this is a direct effect of HFD or secondary to adipose tissue deposition.
Methods: We compared the effects of HFD on obesity-prone and obesity-resistant mice and analyzed lymphatic function in vivo and in vitro.
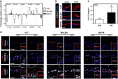
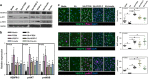
Results: Only obesity-prone mice had impaired lymphatic function, increased perilymphatic inflammation and accumulation of lipid droplets surrounding their lymphatic endothelial cells (LECs). LECs isolated from obesity-prone mice, in contrast to obesity-resistant animals, had decreased expression of VEGFR-3 and Prox1. Exposure of LECs to a long-chain free fatty acid increased cellular apoptosis and decreased VEGFR-3 expression, while inhibition of intracellular inhibitors of VEGFR-3 signaling pathways increased cellular viability.
Conclusions: Collectively, our studies suggest that HFD-induced obesity decreases lymphatic function by increasing perilymphatic inflammation and altering LEC gene expression. Reversal of diminished VEGFR-3 signaling may rescue this phenotype and improve lymphatic function.
Conflict of interest statement
Competing Financial Interests: The authors declare no competing financial interests.
Figures




References
-
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012; 31: 219–230. - PubMed
-
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–126. - PubMed
-
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29: 2959–2971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

