Quantitation of TGF-β proteins in mouse tissues shows reciprocal changes in TGF-β1 and TGF-β3 in normal vs neoplastic mammary epithelium
- PMID: 27203217
- PMCID: PMC5122380
- DOI: 10.18632/oncotarget.9416
Quantitation of TGF-β proteins in mouse tissues shows reciprocal changes in TGF-β1 and TGF-β3 in normal vs neoplastic mammary epithelium
Abstract
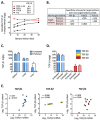
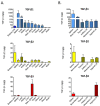
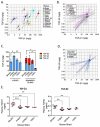
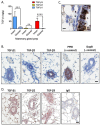
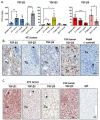
Transforming growth factor-βs (TGF-βs) regulate tissue homeostasis, and their expression is perturbed in many diseases. The three isoforms (TGF-β1, -β2, and -β3) have similar bioactivities in vitro but show distinct activities in vivo. Little quantitative information exists for expression of TGF-β isoform proteins in physiology or disease. We developed an optimized method to quantitate protein levels of the three isoforms, using a Luminex® xMAP®-based multianalyte assay following acid-ethanol extraction of tissues. Analysis of multiple tissues and plasma from four strains of adult mice showed that TGF-β1 is the predominant isoform with TGF-β2 being ~10-fold lower. There were no sex-specific differences in isoform expression, but some tissues showed inter-strain variation, particularly for TGF-β2. The only adult tissue expressing appreciable TGF-β3 was the mammary gland, where its levels were comparable to TGF-β1. In situ hybridization showed the luminal epithelium as the major source of all TGF-β isoforms in the normal mammary gland. TGF-β1 protein was 3-8-fold higher in three murine mammary tumor models than in normal mammary gland, while TGF-β3 protein was 2-3-fold lower in tumors than normal tissue, suggesting reciprocal regulation of these isoforms in mammary tumorigenesis.
Keywords: TGF-β isoforms; mammary gland; mouse tissues; protein; quantitation.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest.
Figures
References
-
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. - PubMed
-
- Hinck AP, O'Connor-McCourt MD. Structures of TGF-beta receptor complexes: implications for function and therapeutic intervention using ligand traps. Curr Pharm Biotechnol. 2011;12:2081–98. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

