Fluctuations in Blood Marginal Zone B-Cell Frequencies May Reflect Migratory Patterns Associated with HIV-1 Disease Progression Status
- PMID: 27203285
- PMCID: PMC4874627
- DOI: 10.1371/journal.pone.0155868
Fluctuations in Blood Marginal Zone B-Cell Frequencies May Reflect Migratory Patterns Associated with HIV-1 Disease Progression Status
Abstract
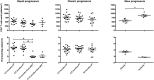
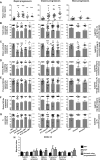
We have previously shown that overexpression of BLyS/BAFF was associated with increased relative frequencies of innate "precursor" marginal zone (MZ)-like B-cells in the blood of HIV-1-infected rapid and classic progressors. However, along with relatively normal BLyS/BAFF expression levels, these cells remain unaltered in elite-controllers (EC), rather, percentages of more mature MZ-like B-cells are decreased in the blood of these individuals. Fluctuations in frequencies of blood MZ-like B-cell populations may reflect migratory patterns associated with disease progression status, suggesting an important role for these cells in HIV-1 pathogenesis. We have therefore longitudinally measured plasma levels of B-tropic chemokines by ELISA-based technology as well as their ligands by flow-cytometry on blood B-cell populations of HIV-1-infected individuals with different rates of disease progression and uninfected controls. Migration potential of B-cell populations from these individuals were determined by chemotaxis assays. We found important modulations of CXCL13-CXCR5, CXCL12-CXCR4/CXCR7, CCL20-CCR6 and CCL25-CCR9 chemokine-axes and increased cell migration patterns in HIV progressors. Interestingly, frequencies of CCR6 expressing cells were significantly elevated within the precursor MZ-like population, consistent with increased migration in response to CCL20. Although we found little modulation of chemokine-axes in EC, cell migration was greater than that observed for uninfected controls, especially for MZ-like B-cells. Overall the immune response against HIV-1 may involve recruitment of MZ-like B-cells to peripheral sites. Moreover, our findings suggest that "regulated" attraction of these cells in a preserved BLyS/BAFF non-inflammatory environment, such as encountered in EC could be beneficial to the battle and even control of HIV.
Conflict of interest statement
Figures





Similar articles
-
Blood B Lymphocyte Stimulator (BLyS)/BAFF levels may reflect natural immunity to HIV in highly exposed uninfected Beninese Commercial Sex Workers.Sci Rep. 2016 Aug 26;6:32318. doi: 10.1038/srep32318. Sci Rep. 2016. PMID: 27561453 Free PMC article.
-
High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans.Blood. 2011 Jan 6;117(1):145-55. doi: 10.1182/blood-2010-08-301887. Epub 2010 Sep 24. Blood. 2011. PMID: 20870901
-
IL-10 and lymphotoxin-α expression profiles within marginal zone-like B-cell populations are associated with control of HIV-1 disease progression.PLoS One. 2014 Jul 8;9(7):e101949. doi: 10.1371/journal.pone.0101949. eCollection 2014. PLoS One. 2014. PMID: 25003989 Free PMC article.
-
Marginal Zone B-Cell Populations and Their Regulatory Potential in the Context of HIV and Other Chronic Inflammatory Conditions.Int J Mol Sci. 2022 Mar 21;23(6):3372. doi: 10.3390/ijms23063372. Int J Mol Sci. 2022. PMID: 35328792 Free PMC article. Review.
-
Natural Immunity to HIV: A Template for Vaccine Strategies.Viruses. 2018 Apr 23;10(4):215. doi: 10.3390/v10040215. Viruses. 2018. PMID: 29690575 Free PMC article. Review.
Cited by
-
Streptococcus pneumoniae Nasopharyngeal Carriage among PCV-10-Vaccinated HIV-1-Infected Children with Maintained Serological Memory in Ethiopia.Pathogens. 2020 Feb 25;9(3):159. doi: 10.3390/pathogens9030159. Pathogens. 2020. PMID: 32106620 Free PMC article.
-
The Role of BAFF System Molecules in Host Response to Pathogens.Clin Microbiol Rev. 2017 Oct;30(4):991-1014. doi: 10.1128/CMR.00046-17. Clin Microbiol Rev. 2017. PMID: 28855265 Free PMC article. Review.
-
Editorial: Gateway to monocyte entry into the brain: CXCR7, the new orchestra conductor.J Leukoc Biol. 2017 Nov;102(5):1155-1157. doi: 10.1189/jlb.3CE0717-273R. J Leukoc Biol. 2017. PMID: 29093133 Free PMC article.
-
The Role of CXCL13 in Antibody Responses to HIV-1 Infection and Vaccination.Front Immunol. 2021 Feb 25;12:638872. doi: 10.3389/fimmu.2021.638872. eCollection 2021. Front Immunol. 2021. PMID: 33732259 Free PMC article. Review.
-
Blood B Lymphocyte Stimulator (BLyS)/BAFF levels may reflect natural immunity to HIV in highly exposed uninfected Beninese Commercial Sex Workers.Sci Rep. 2016 Aug 26;6:32318. doi: 10.1038/srep32318. Sci Rep. 2016. PMID: 27561453 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

