Sialic acid removal from dendritic cells improves antigen cross-presentation and boosts anti-tumor immune responses
- PMID: 27203391
- PMCID: PMC5173042
- DOI: 10.18632/oncotarget.9419
Sialic acid removal from dendritic cells improves antigen cross-presentation and boosts anti-tumor immune responses
Abstract
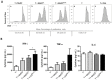
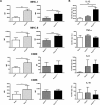
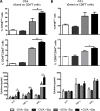
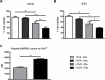
Dendritic cells (DCs) hold promise for anti-cancer immunotherapy. However, clinically, their efficiency is limited and novel strategies to improve DC-mediated anti-tumor responses are needed. Human DCs display high content of sialic acids, which inhibits their maturation and co-stimulation capacity. Here, we aimed to understand whether exogenous desialylation of DCs improves their anti-tumor immunity. Compared to fully sialylated DCs, desialylated human DCs loaded with tumor-antigens showed enhanced ability to induce autologous T cells to proliferate, to secrete Th1 cytokines, and to specifically induce tumor cell apoptosis. Desialylated DCs showed an increased expression of MHC-I and -II, co-stimulatory molecules and an augmented secretion of IL-12. Desialylated HLA-A*02:01 DCs pulsed with gp100 peptides displayed enhanced peptide presentation through MHC-I, resulting in higher activation ofgp100280-288 specific CD8+ cytotoxic T cells. Desialylated murine DCs also exhibited increased MHC and co-stimulatory molecules and higher antigen cross-presentation via MHC-I. These DCs showed higher ability to activate antigen-specific CD4+ and CD8+ T cells, and to specifically induce tumor cell apoptosis. Collectively, our data demonstrates that desialylation improves DCs' ability to elicit T cell-mediated anti-tumor activity, due to increased MHC-I expression and higher antigen presentation via MHC-I. Sialidase treatment of DCs may represent a technology to improve the efficacy of antigen loaded-DC-based vaccines for anti-cancer immunotherapy.
Keywords: Th1-polarization; anti-tumor immunity; antigen cross-presentation; dendritic cells; sialic acid.
Conflict of interest statement
All authors declare no competing financial interests.
Figures



References
-
- Strioga MM, Felzmann T, Powell DJ, Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J, Schijns VE. Therapeutic dendritic cell-based cancer vaccines: the state of the art. Crit Rev Immunol. 2013;33:489–547. - PubMed
-
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–267. - PubMed
-
- Bhargava A, Mishra D, Banerjee S, Mishra PK. Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Immunotherapy. 2012;4:703–718. - PubMed
-
- Dawson N. Immunotherapeutic Approaches in Prostate Cancer: PROVENGE. Clinical advances in hematology & oncology. 2010;8:419–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

