Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study
- PMID: 27203434
- PMCID: PMC4874598
- DOI: 10.1371/journal.pone.0153052
Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study
Abstract
Purpose: To compare the effectiveness of bevacizumab and ranibizumab in the treatment of exudative age-related macular degeneration (AMD).
Design: Multicentre, randomized, controlled, double-masked clinical trial in 327 patients. The non-inferiority margin was 4 letters.
Patients: Patients ≥ 60 years of age with primary or recurrent sub- or juxtafoveal choroidal neovascularization (CNV) secondary to AMD with a total area of CNV < 12 disc areas and a best corrected visual acuity (BCVA) score between 20 and 78 letters on an EDTRS like chart in the study eye.
Methods: Monthly intravitreal injections with 1.25 mg bevacizumab or 0.5 mg ranibizumab were given during one year. Intention to treat with last observation carried forward analysis was performed.
Main outcome measures: Primary outcome was the change in BCVA in the study eye from baseline to 12 months.
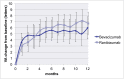
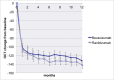
Results: The mean gain in BCVA was 5.1 (±14.1) letters in the bevacizumab group (n = 161) and 6.4 (±12.2) letters in the ranibizumab group (n = 166) (p = 0.37). The lower limit of the 95% confidence interval of the difference in BCVA gain was 3.72. The response to bevacizumab was more varied; 24% of patients showed a gain of ≥15 letters, 11% a loss of ≥15 letters and 65% a gain or loss < 15 letters compared to 19%, 5% and 76% respectively for ranibizumab (p = 0.038). No significant differences in absolute CRT and CRT change (p = 0.13) or in the presence of subretinal or intraretinal fluid (p = 0.14 and 0.10, respectively) were observed. However, the presence of any fluid on SD-OCT (subretinal and/or intraretinal) differed significantly (p = 0.020), with definite fluid on SD-OCT in 45% of the patients for bevacizumab versus 31% for ranibizumab. The occurrence of serious adverse events and adverse events was similar, with 34 SAEs and 256 AEs in the bevacizumab group and 37 SAEs and 299 AEs in the ranibizumab group (p = 0.87 and p = 0.48, respectively).
Conclusions: Bevacizumab was not inferior to ranibizumab. The response to bevacizumab was more varied with higher percentages of both gainers and losers and more frequently observed retinal fluid on SD-OCT at 12 months when compared to the ranibizumab group.
Trial registration: Trialregister.nl NTR1704.
Conflict of interest statement
Figures

References
-
- Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. (1995) The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 102: 205–210. - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119: 1399–1411. S0161-6420(12)00358-2 [pii]; 10.1016/j.ophtha.2012.04.015 - DOI - PubMed
-
- Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, Egger S, et al. (2013) A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol 97: 266–271. bjophthalmol-2012-302391 [pii]; 10.1136/bjophthalmol-2012-302391 - DOI - PubMed
-
- Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, et al. (2013) Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 120: 2300–2309. S0161-6420(13)00524-1 [pii]; 10.1016/j.ophtha.2013.06.020 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

