Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers
- PMID: 27203445
- PMCID: PMC5023393
- DOI: 10.1038/mt.2016.101
Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers
Abstract
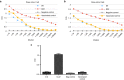
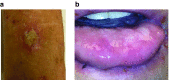
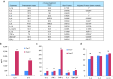
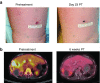
We have conducted a phase 1 study of intravenous vvDD, a Western Reserve strain oncolytic vaccinia virus, on 11 patients with standard treatment-refractory advanced colorectal or other solid cancers. The primary endpoints were maximum tolerated dose and associated toxicity while secondary endpoints were pharmacokinetics, pharmacodynamics, immune responses, and antitumor activity. No dose-limiting toxicities and treatment related severe adverse events were observed. The most common adverse events were grades 1/2 flu-like symptoms. Virus genomes were detectable in the blood 15-30 minutes after virus administration in a dose-dependent manner. There was evidence of a prolonged virus replication in tumor tissues in two patients, but no evidence of virus replication in non-tumor tissues, except a healed injury site and an oral thrush. Over 100-fold of anti-viral antibodies were induced in patients' sera. A strong induction of inflammatory and Th1, but not Th2 cytokines, suggested a potent Th1-mediated immunity against the virus and possibly the cancer. One patient showed a mixed response on PET-CT with resolution of some liver metastases, and another patient with cutaneous melanoma demonstrated clinical regression of some lesions. Given the confirmed safety, further trials evaluating intravenous vvDD in combination with therapeutic transgenes, immune checkpoint blockade or complement inhibitors, are warranted.
Figures

Similar articles
-
First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity.Mol Ther. 2015 Jan;23(1):202-14. doi: 10.1038/mt.2014.194. Epub 2014 Oct 8. Mol Ther. 2015. PMID: 25292189 Free PMC article. Clinical Trial.
-
Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer.Mol Ther. 2015 Sep;23(9):1532-40. doi: 10.1038/mt.2015.109. Epub 2015 Jun 15. Mol Ther. 2015. PMID: 26073886 Free PMC article. Clinical Trial.
-
Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer.Mol Ther. 2012 Jan;20(1):221-9. doi: 10.1038/mt.2011.230. Epub 2011 Nov 1. Mol Ther. 2012. PMID: 22044933 Free PMC article.
-
Oncolytic virus therapy: A new era of cancer treatment at dawn.Cancer Sci. 2016 Oct;107(10):1373-1379. doi: 10.1111/cas.13027. Epub 2016 Sep 9. Cancer Sci. 2016. PMID: 27486853 Free PMC article. Review.
-
Oncolytic Viruses for the Treatment of Metastatic Melanoma.Curr Treat Options Oncol. 2020 Mar 19;21(4):26. doi: 10.1007/s11864-020-0718-2. Curr Treat Options Oncol. 2020. PMID: 32266483 Review.
Cited by
-
Design Strategies and Precautions for Using Vaccinia Virus in Tumor Virotherapy.Vaccines (Basel). 2022 Sep 17;10(9):1552. doi: 10.3390/vaccines10091552. Vaccines (Basel). 2022. PMID: 36146629 Free PMC article. Review.
-
Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics.J Immunother Cancer. 2019 Jan 9;7(1):6. doi: 10.1186/s40425-018-0495-7. J Immunother Cancer. 2019. PMID: 30626434 Free PMC article. Review.
-
Vaccinia virus-based vaccines confer protective immunity against SARS-CoV-2 virus in Syrian hamsters.PLoS One. 2021 Sep 9;16(9):e0257191. doi: 10.1371/journal.pone.0257191. eCollection 2021. PLoS One. 2021. PMID: 34499677 Free PMC article.
-
Modeling oncolytic virus dynamics in the tumor microenvironment using zebrafish.Cancer Gene Ther. 2021 Aug;28(7-8):769-784. doi: 10.1038/s41417-020-0194-7. Epub 2020 Jul 10. Cancer Gene Ther. 2021. PMID: 32647136
-
Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase.Biomedicines. 2020 Oct 16;8(10):426. doi: 10.3390/biomedicines8100426. Biomedicines. 2020. PMID: 33081279 Free PMC article.
References
-
- Lichty, BD, Breitbach, CJ, Stojdl, DF and Bell, JC (2014). Going viral with cancer immunotherapy. Nat Rev Cancer 14: 559–567. - PubMed
-
- Andtbacka, RH, Kaufman, HL, Collichio, F, Amatruda, T, Senzer, N, Chesney, J et al. (2015). Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 33: 2780–2788. - PubMed
-
- Ganly, I, Kirn, D, Eckhardt, G, Rodriguez, GI, Soutar, DS, Otto, R et al. (2000). A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res 6: 798–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

