Drug conjugated nanoparticles activated by cancer cell specific mRNA
- PMID: 27203672
- PMCID: PMC5122386
- DOI: 10.18632/oncotarget.9430
Drug conjugated nanoparticles activated by cancer cell specific mRNA
Abstract
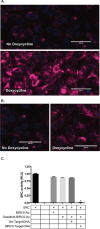
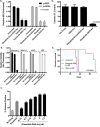
We describe a customizable approach to cancer therapy in which a gold nanoparticle (Au-NP) delivers a drug that is selectively activated within the cancer cell by the presence of an mRNA unique to the cancer cell. Fundamental to this approach is the observation that the amount of drug released from the Au-NP is proportional to both the presence and abundance of the cancer cell specific mRNA in a cell. As proof-of-principle, we demonstrate both the efficient delivery and selective release of the multi-kinase inhibitor dasatinib from Au-NPs in leukemia cells with resulting efficacy in vitro and in vivo. Furthermore, these Au-NPs reduce toxicity against hematopoietic stem cells and T-cells. This approach has the potential to improve the therapeutic efficacy of a drug and minimize toxicity while being highly customizable with respect to both the cancer cell specific mRNAs targeted and drugs activated.
Keywords: anti-sense; drug delivery; gold nanoparticles; leukemia; molecularly targeted therapy.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

