Detection and manipulation of live antigen-expressing cells using conditionally stable nanobodies
- PMID: 27205882
- PMCID: PMC4922844
- DOI: 10.7554/eLife.15312
Detection and manipulation of live antigen-expressing cells using conditionally stable nanobodies
Abstract
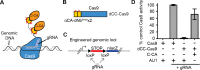
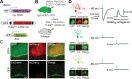
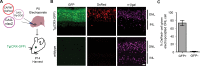
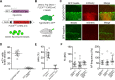
The ability to detect and/or manipulate specific cell populations based upon the presence of intracellular protein epitopes would enable many types of studies and applications. Protein binders such as nanobodies (Nbs) can target untagged proteins (antigens) in the intracellular environment. However, genetically expressed protein binders are stable regardless of antigen expression, complicating their use for applications that require cell-specificity. Here, we created a conditional system in which the stability of an Nb depends upon an antigen of interest. We identified Nb framework mutations that can be used to rapidly create destabilized Nbs. Fusion of destabilized Nbs to various proteins enabled applications in living cells, such as optogenetic control of neural activity in specific cell types in the mouse brain, and detection of HIV-infected human cells by flow cytometry. These approaches are generalizable to other protein binders, and enable the rapid generation of single-polypeptide sensors and effectors active in cells expressing specific intracellular epitopes.
Keywords: GFP; HIV-1; antibody engineering; developmental biology; human; mouse; nanobodies; neuroscience; stem cells.
Conflict of interest statement
JCYT, ED, SW and CLC: Submitted a patent application regarding destabilized nanobodies. International Application No. PCT/US2016/027749 Priority: US Prov. Appl. No. 62/148,595.
The other authors declare that no competing interests exist.
Figures


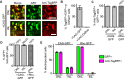
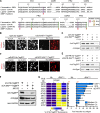

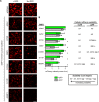

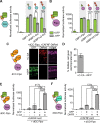



Comment in
-
Falling apart.Elife. 2016 Jun 27;5:e18203. doi: 10.7554/eLife.18203. Elife. 2016. PMID: 27345573 Free PMC article.
References
-
- Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SP, Sabatini B, Cepko CL. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

