Characterisation of Candida within the Mycobiome/Microbiome of the Lower Respiratory Tract of ICU Patients
- PMID: 27206014
- PMCID: PMC4874575
- DOI: 10.1371/journal.pone.0155033
Characterisation of Candida within the Mycobiome/Microbiome of the Lower Respiratory Tract of ICU Patients
Abstract
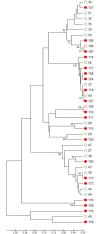
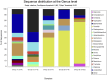
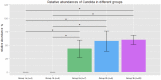
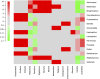
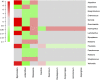
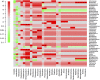
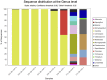
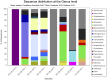
Whether the presence of Candida spp. in lower respiratory tract (LRT) secretions is a marker of underlying disease, intensive care unit (ICU) treatment and antibiotic therapy or contributes to poor clinical outcome is unclear. We investigated healthy controls, patients with proposed risk factors for Candida growth in LRT (antibiotic therapy, ICU treatment with and without antibiotic therapy), ICU patients with pneumonia and antibiotic therapy and candidemic patients (for comparison of truly invasive and colonizing Candida spp.). Fungal patterns were determined by conventional culture based microbiology combined with molecular approaches (next generation sequencing, multilocus sequence typing) for description of fungal and concommitant bacterial microbiota in LRT, and host and fungal biomarkes were investigated. Admission to and treatment on ICUs shifted LRT fungal microbiota to Candida spp. dominated fungal profiles but antibiotic therapy did not. Compared to controls, Candida was part of fungal microbiota in LRT of ICU patients without pneumonia with and without antibiotic therapy (63% and 50% of total fungal genera) and of ICU patients with pneumonia with antibiotic therapy (73%) (p<0.05). No case of invasive candidiasis originating from Candida in the LRT was detected. There was no common bacterial microbiota profile associated or dissociated with Candida spp. in LRT. Colonizing and invasive Candida strains (from candidemic patients) did not match to certain clades withdrawing the presence of a particular pathogenic and invasive clade. The presence of Candida spp. in the LRT rather reflected rapidly occurring LRT dysbiosis driven by ICU related factors than was associated with invasive candidiasis.
Conflict of interest statement
Figures



References
-
- Baum GL. The Significance of Candida albicans in Human Sputum. The Significance of Candida albicans in Human Sputum. New Engl J Med 1960; 263:70–3. - PubMed
-
- el-Ebiary M, Torres A, Fàbregas N, la Bellacasa de JP, González J, Ramirez J, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156: 583–590. 10.1164/ajrccm.156.2.9612023 - DOI - PubMed
-
- Rello J, Esandi ME, Díaz E, Mariscal D, Gallego M, Vallès J. The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients. Chest. 1998;114: 146–149. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

