Microfluidic manufacture of rt-PA -loaded echogenic liposomes
- PMID: 27206512
- PMCID: PMC4920071
- DOI: 10.1007/s10544-016-0072-0
Microfluidic manufacture of rt-PA -loaded echogenic liposomes
Abstract
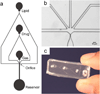
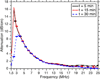
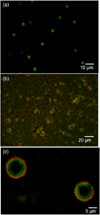
Echogenic liposomes (ELIP), loaded with recombinant tissue-type plasminogen activator (rt-PA) and microbubbles that act as cavitation nuclei, are under development for ultrasound-mediated thrombolysis. Conventional manufacturing techniques produce a polydisperse rt-PA-loaded ELIP population with only a small percentage of particles containing microbubbles. Further, a polydisperse population of rt-PA-loaded ELIP has a broadband frequency response with complex bubble dynamics when exposed to pulsed ultrasound. In this work, a microfluidic flow-focusing device was used to generate monodisperse rt-PA-loaded ELIP (μtELIP) loaded with a perfluorocarbon gas. The rt-PA associated with the μtELIP was encapsulated within the lipid shell as well as intercalated within the lipid shell. The μtELIP had a mean diameter of 5 μm, a resonance frequency of 2.2 MHz, and were found to be stable for at least 30 min in 0.5 % bovine serum albumin. Additionally, 35 % of μtELIP particles were estimated to contain microbubbles, an order of magnitude higher than that reported previously for batch-produced rt-PA-loaded ELIP. These findings emphasize the advantages offered by microfluidic techniques for improving the encapsulation efficiency of both rt-PA and perflurocarbon microbubbles within echogenic liposomes.
Keywords: Echogenic liposomes; Microfluidic flow-focusing; Recombinant tissue-type plasminogen activator; Stroke treatment; Ultrasound-mediated thrombolysis.
Figures





References
-
- Akers MJ, Defelippis MR. In: Pharmaceutical Formulation Development of Peptides and Proteins. Frokjaer S, Hovgaard L, editors. Philadelphia: Taylor and Francis; 2000. pp. 145–177.
-
- Alexandrov AV, Grotta JC. Neurology. 2002;59(6):862–867. - PubMed
-
- Alexandrov AV, Wojner AW, Grotta JC. J. Neuroimaging. 2004;14(2):108–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

