Pathway-Specific Dopamine Abnormalities in Schizophrenia
- PMID: 27206569
- PMCID: PMC5177794
- DOI: 10.1016/j.biopsych.2016.03.2104
Pathway-Specific Dopamine Abnormalities in Schizophrenia
Abstract
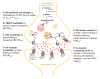
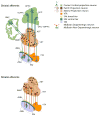
In light of the clinical evidence implicating dopamine in schizophrenia and the prominent hypotheses put forth regarding alterations in dopaminergic transmission in this disease, molecular imaging has been used to examine multiple aspects of the dopaminergic system. We review the imaging methods used and compare the findings across the different molecular targets. Findings have converged to suggest early dysregulation in the striatum, especially in the rostral caudate, manifesting as excess synthesis and release. Recent data showed deficit extending to most cortical regions and even to other extrastriatal subcortical regions not previously considered to be "hypodopaminergic" in schizophrenia. These findings yield a new topography for the dopaminergic dysregulation in schizophrenia. We discuss the dopaminergic innervation within the individual projection fields to provide a topographical map of this dual dysregulation and explore potential cellular and circuit-based mechanisms for brain region-dependent alterations in dopaminergic parameters. This refined knowledge is essential to better guide translational studies and efforts in early drug development.
Keywords: Cortex; Dopamine; Neuroanatomy; PET imaging; Schizophrenia; Striatum.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Kegeles has received research support from Amgen. Dr. Slifstein has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka and has provided consultation for Amgen. Dr. Abi-Dargham has received research support from Takeda and Forest Pharmaceuticals and has served on advisory boards for Roche, Forum, and Otsuka. Drs. Moore, Chohan, and Weinstein report no biomedical financial interests or potential conflicts of interest.
Figures


References
-
- Rossum V. The significance of dopamine receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Therapy. 1966;160:492–494. - PubMed
-
- Carlsson A. Antipsychotic drugs, neurotransmitters, and schizophrenia. The American journal of psychiatry. 1978;135:165–173. - PubMed
-
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. - PubMed
-
- Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–433. - PubMed
-
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophrenia Bull. 1988;14:157–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

