Clinical-scale expansion of mesenchymal stromal cells: a large banking experience
- PMID: 27207011
- PMCID: PMC4875672
- DOI: 10.1186/s12967-016-0892-y
Clinical-scale expansion of mesenchymal stromal cells: a large banking experience
Abstract
Background: Mesenchymal stromal cells (MSC) are largely investigated in clinical trials aiming to control inappropriate immune reactions (GVHD, Crohn's disease, solid organ transplantation). As the percentage of MSC precursors in bone marrow is very low, these must be expanded in vitro to obtain therapeutic cell doses. We describe here the constitution of an allogeneic human third-party MSC bank from screened healthy volunteer donors in compliance with quality specifications and ISCT-release criteria and report follow-up of different aspects of this activity since 2007.
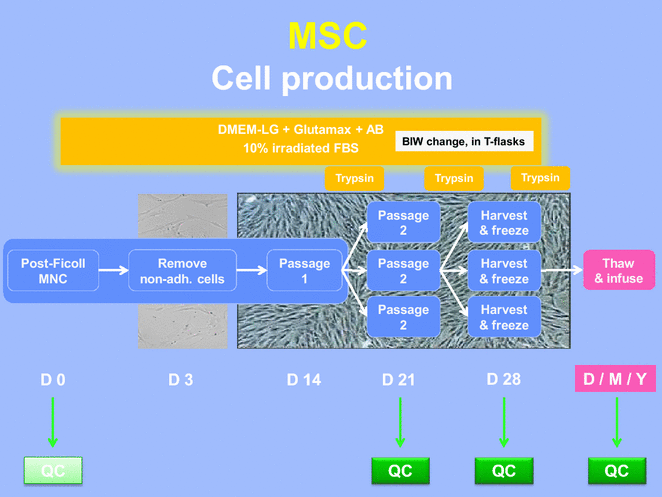
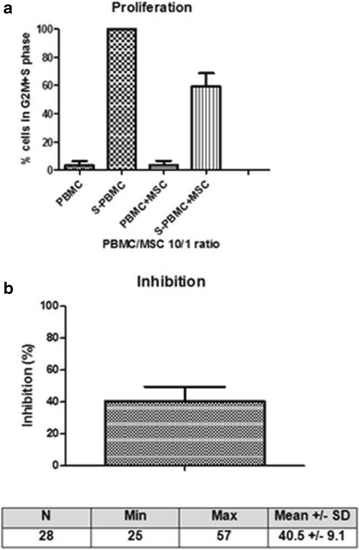
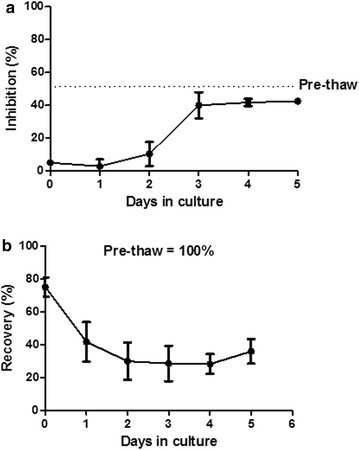
Methods: 68 clinical-grade large-scale MSC cultures were completed and analyzed. The whole process was described, including volunteer donor screening, bone marrow collection, mononuclear cell isolation and expansion over 4 weeks, harvesting, cryopreservation, release, administration and quality controls of the cells (including microbiology, phenotype, and potency assays).
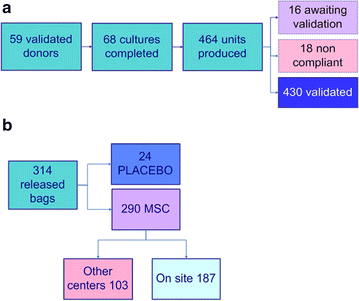
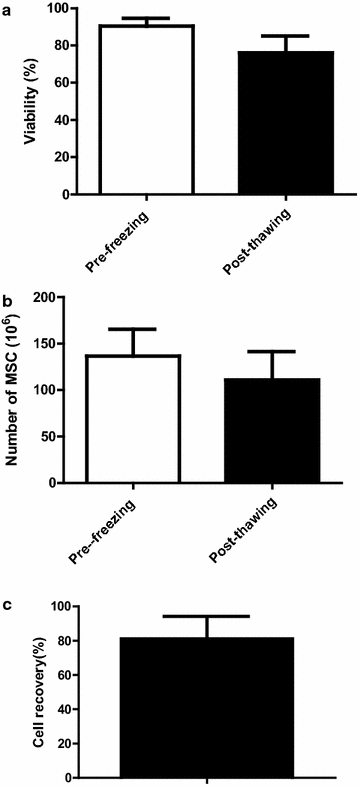
Results: From 59 validated donors, 68 cultures were completed (mean of final yields: 886 × 10(6) cells/culture) and a total of 464 MSC aliquots have been produced and stored in liquid nitrogen (mean of 132.8 × 10(6) cells/bag). Each MSC batch underwent extensive testing to verify its conformity with EBMT and ISCT release criteria and was individually validated. As of June 1 2015, 314 bags have been released and infused to patients included in 6 different clinical protocols. All thawed MSC units satisfied to release criteria and no infusion-related toxicity was reported.
Conclusion: In conclusion, despite low passage cultures, we have been able to create an allogeneic "off-the-shelf" MSC bank with a large number of frozen aliquots and report here an efficient clinical-grade MSC banking activity in place for more than 7 years. Our challenge now is to produce MSC in compliance with good manufacturing practices (GMP) as, in the meantime, MSC have become considered as advanced therapy medicinal products (ATMP). Another significant challenge remains the development of relevant potency assay.
Keywords: Banking; Clinical-grade; GMP; MSC; Mesenchymal stem cells.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

