Implementation plans included in World Health Organisation guidelines
- PMID: 27207104
- PMCID: PMC4875699
- DOI: 10.1186/s13012-016-0440-4
Implementation plans included in World Health Organisation guidelines
Abstract
Background: The implementation of high-quality guidelines is essential to improve clinical practice and public health. The World Health Organisation (WHO) develops evidence-based public health and other guidelines that are used or adapted by countries around the world. Detailed implementation plans are often necessary for local policymakers to properly use the guidelines developed by WHO. This paper describes the plans for guideline implementation reported in WHO guidelines and indicates which of these plans are evidence-based.
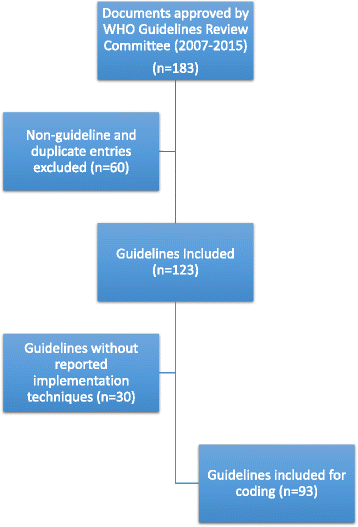
Methods: We conducted a content analysis of the implementation sections of WHO guidelines approved by the WHO guideline review committee between December 2007 and May 2015. The implementation techniques reported in each guideline were coded according to the Cochrane Collaboration's Effective Practice and Organisation of Care (EPOC) taxonomy and classified as passive, active or policy strategies. The frequencies of implementation techniques are reported.
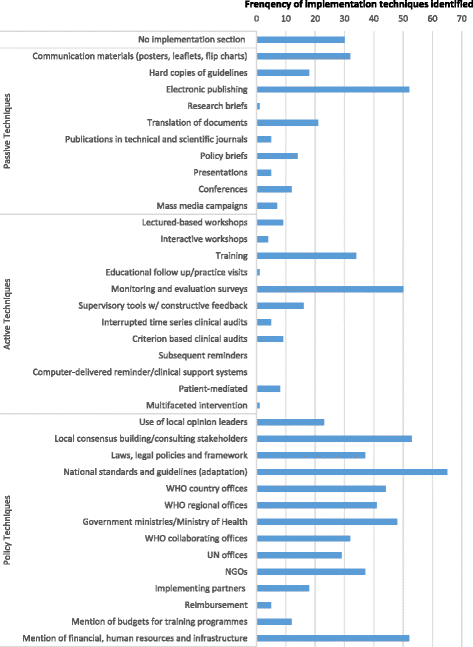
Results: The WHO guidelines (n = 123) analysed mentioned implementation techniques 800 times, although most mentioned implementation techniques very briefly, if at all. Passive strategies (21 %, 167/800) and general policy strategies (62 %, 496/800) occurred most often. Evidence-based active implementation methods were generally neglected with no guideline mentioning reminders (computerised or paper) and only one mentioning a multifaceted approach. Many guidelines contained implementation sections that were identical to those used in older guidelines produced by the same WHO technical unit.
Conclusions: The prevalence of passive and policy-based implementation techniques as opposed to evidence-based active techniques suggests that WHO guidelines should contain stronger guidance for implementation. This could include structured and increased detail on implementation considerations, accompanying or linked documents that provide information on what is needed to contextualise or adapt a guideline and specific options from among evidence-based implementation strategies.
Keywords: Clinical practice guidelines; Guidelines; Guidelines Review Committee; Implementation; Implementation strategies; Implementation techniques; WHO.
Figures
Similar articles
-
Variations in processes for guideline adaptation: a qualitative study of World Health Organization staff experiences in implementing guidelines.BMC Public Health. 2020 Nov 23;20(1):1758. doi: 10.1186/s12889-020-09812-0. BMC Public Health. 2020. PMID: 33228608 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Nurses' adoption of diabetes clinical practice guidelines in primary care and the impacts on patient outcomes and safety: An integrative review.Int J Nurs Stud. 2024 Jun;154:104747. doi: 10.1016/j.ijnurstu.2024.104747. Epub 2024 Mar 6. Int J Nurs Stud. 2024. PMID: 38531197 Review.
-
Implementation strategies used to implement nursing guidelines in daily practice: A systematic review.Int J Nurs Stud. 2020 Nov;111:103748. doi: 10.1016/j.ijnurstu.2020.103748. Epub 2020 Aug 18. Int J Nurs Stud. 2020. PMID: 32961463
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Factors shaping the implementation of the SAFE strategy for trachoma using the Consolidated Framework for Implementation Research: a systematic review.Glob Health Action. 2019;12(1):1570646. doi: 10.1080/16549716.2019.1570646. Glob Health Action. 2019. PMID: 30773102 Free PMC article.
-
The advantages and limitations of guideline adaptation frameworks.Implement Sci. 2018 May 29;13(1):72. doi: 10.1186/s13012-018-0763-4. Implement Sci. 2018. PMID: 29843737 Free PMC article.
-
Assessing unConventional Evidence (ACE) tool: development and content of a tool to assess the strengths and limitations of 'unconventional' source materials.Health Res Policy Syst. 2024 Jan 2;22(1):2. doi: 10.1186/s12961-023-01080-9. Health Res Policy Syst. 2024. PMID: 38167048 Free PMC article.
-
Study protocol for a cluster-randomized trial to compare human papillomavirus based cervical cancer screening in community-health campaigns versus health facilities in western Kenya.BMC Cancer. 2017 Dec 6;17(1):826. doi: 10.1186/s12885-017-3818-z. BMC Cancer. 2017. PMID: 29207966 Free PMC article.
-
Variations in processes for guideline adaptation: a qualitative study of World Health Organization staff experiences in implementing guidelines.BMC Public Health. 2020 Nov 23;20(1):1758. doi: 10.1186/s12889-020-09812-0. BMC Public Health. 2020. PMID: 33228608 Free PMC article.
References
-
- Oxman AD, Lavis JN, Fretheim A. Use of evidence in WHO recommendations. The Lancet.369(9576):1883–9. doi:10.1016/S0140-6736(07)60675-8. - PubMed
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- World Health Organization . WHO Handbook for Guideline Development. 2. Geneva, Switzerland: WHO Press; 2014.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources