In vivo photoacoustic microscopy of human cuticle microvasculature with single-cell resolution
- PMID: 27207113
- PMCID: PMC5998605
- DOI: 10.1117/1.JBO.21.5.056004
In vivo photoacoustic microscopy of human cuticle microvasculature with single-cell resolution
Abstract
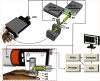
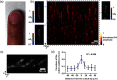
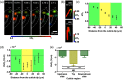
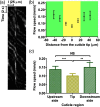
As a window on the microcirculation, human cuticle capillaries provide rich information about the microvasculature, such as its morphology, density, dimensions, or even blood flow speed. Many imaging technologies have been employed to image human cuticle microvasculature. However, almost none of these techniques can noninvasively observe the process of oxygen release from single red blood cells (RBCs), an observation which can be used to study healthy tissue functionalities or to diagnose, stage, or monitor diseases. For the first time, we adapted single-cell resolution photoacoustic (PA) microscopy (PA flowoxigraphy) to image cuticle capillaries and quantified multiple functional parameters. Our results show more oxygen release in the curved cuticle tip region than in other regions of a cuticle capillary loop, associated with a low of RBC flow speed in the tip region. Further analysis suggests that in addition to the RBC flow speed, other factors, such as the drop of the partial oxygen pressure in the tip region, drive RBCs to release more oxygen in the tip region.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

