Pharmaceutical expenditure on drugs for rare diseases in Canada: a historical (2007-13) and prospective (2014-18) MIDAS sales data analysis
- PMID: 27207271
- PMCID: PMC4875716
- DOI: 10.1186/s13023-016-0450-y
Pharmaceutical expenditure on drugs for rare diseases in Canada: a historical (2007-13) and prospective (2014-18) MIDAS sales data analysis
Abstract
Background: Health Canada has defined rare diseases as life-threatening, seriously debilitating, or serious chronic conditions affecting a very small number of patients (~1 in 2,000 persons). An estimated 9 % of Canadians suffer from a rare disease. Drugs treating rare diseases (DRDs) are also known as orphan drugs. While Canada is currently developing an orphan drug framework, in the United States (US), the Orphan Drug Act (ODA) of 1983 established incentives for the development of orphan drugs. This study measured total annual expenditure of orphan drugs in Canada (2007-13) and estimated future (2014-18) orphan drug expenditure.
Methods: Orphan drugs approved by the US Food and Drug Administration (FDA) in the US were used as a proxy for the orphan drug landscape in Canada. Branded, orphan drugs approved by the FDA between 1983 through 2013 were identified (N = 356 unique products). Only US orphan drugs with the same orphan indication(s) approved in Canada were included in the analysis. Adjustment via an indication factoring was applied to products with both orphan and non-orphan indications using available data sources to isolate orphan-indication sales. The IMS Health MIDAS database of audited biopharmaceutical sales was utilized to measure total orphan drug expenditure, calculated annually from 2007-2013 and evaluated as a proportion of total annual pharmaceutical drug expenditure (adjusted to 2014 CAD).
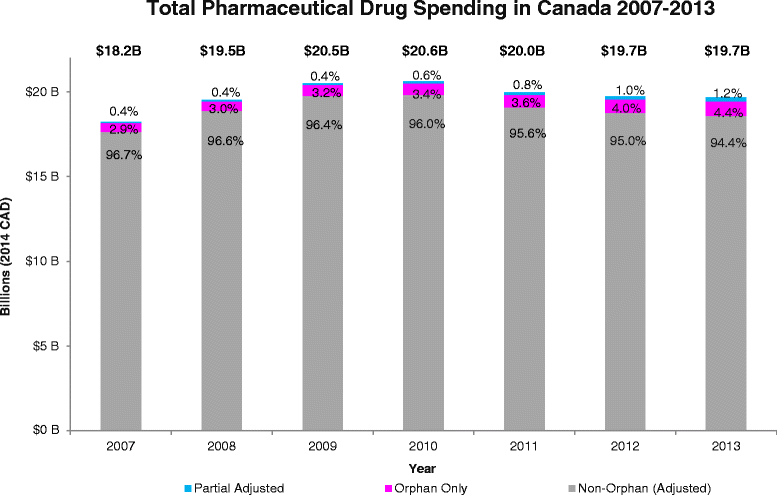
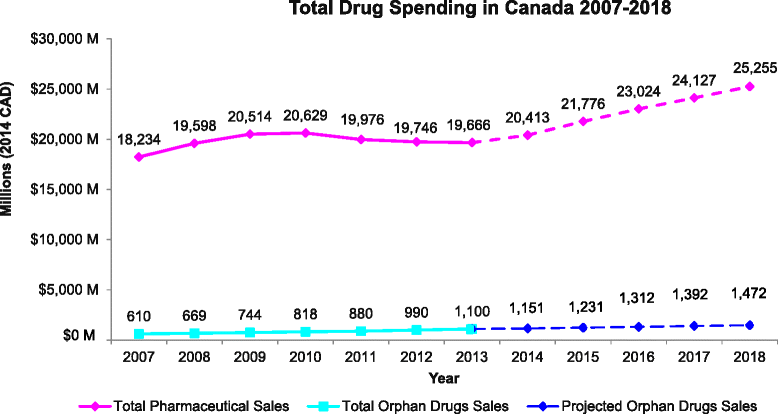
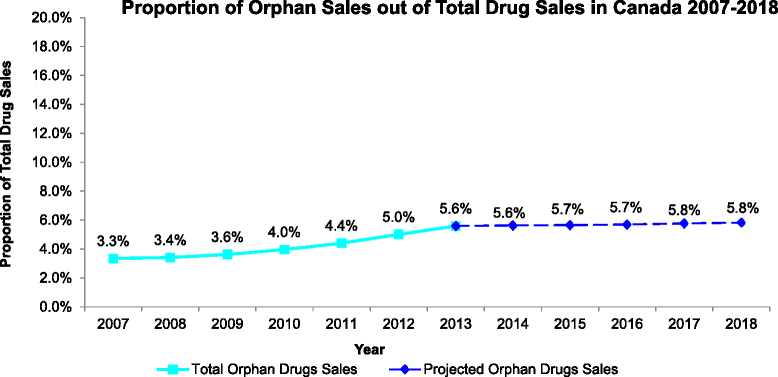
Results: Between 2007 and 2013, expenditure was measured for a final N = 147 orphan drugs. Orphan drug expenditure totaled $610.2 million (M) in 2007 and $1,100.0 M in 2013, representing 3.3- 5.6 % of total Canadian pharmaceutical drug expenditure in 2007-2013, respectively. Future trend analysis suggests orphan drug expenditure will remain under 6 % of total expenditure in 2014-18.
Conclusions: While the number of available orphan drugs and associated expenditure increased over time, access remains an issue, and from the perspectives of society and equity, overall spending on orphan drugs is lower relative to the number of patients affected with an orphan disease in Canada. The overall budget impact of orphan drugs is small and fairly stable relative to total pharmaceutical expenditure. Concerns that growth in orphan drug expenditure may lead to unsustainable drug expenditure do not appear to be justified.
Keywords: Cost of health care; Health economics; Health spending; Orphan drugs; Pharmaceuticals; Rare diseases.
Figures
References
-
- Initial draft discussion document for a Canadian orphan drug regulatory framework. Health Canada, Office of Legislative and Regulatory Modernization. 2012. http://www.orpha.net/national/data/CA-EN/www/uploads/Initial-Draft-Discu.... Accessed 1 Dec 2015.
-
- Critchley W. Orphan drugs update – Canada. Presented at: FDLI Conference. 2014 May 14–15; Toronto, CA. http://www.fdli.org/docs/canadian/critchley-fdli-may-14-2014.pdf?sfvrsn=0. Accessed 1 Dec 2015.
-
- Lee DK, Wong B. An Orphan Drug Framework (ODF) for Canada. J Popul Ther Clin Pharmacol. 2014;21:e42–6. - PubMed
-
- Electronic code of federal regulations title 21 food and drugs part 316 orphan drugs. U.S. Government Publishing Office. http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID=238362420de6b80c19b2a9c4.... Accessed 1 Dec 2015.
-
- Search orphan drug designations and approvals. U.S. Food and Drug Administration. www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed 28 Jul 2014.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous