Hsp70 exerts oncogenic activity in the Apc mutant Min mouse model
- PMID: 27207671
- PMCID: PMC6276941
- DOI: 10.1093/carcin/bgw056
Hsp70 exerts oncogenic activity in the Apc mutant Min mouse model
Abstract
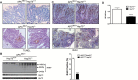
Colorectal cancer (CRC) develops from colonic epithelial cells that lose expression of key tumor suppressor genes and/or gain expression of proproliferative and antiapoptotic genes like heat shock protein 70 (Hsp70). Heat shock protein 70 is overexpressed in CRC, but it is not known whether this is in response to the proteotoxic stress induced by transformation, or if it contributes to the process of transformation itself. Here, using the Apc (Min/+) mouse model of CRC, we show that Hsp70 regulates mitogenic signaling in intestinal epithelial cells through stabilization of proteins involved in the receptor tyrosine kinase (RTK) and WNT signaling pathways. Loss of Hsp70 reduced tumor size with decreased proliferation and increased tumor cell death. Hsp70 loss also led to decreased expression of ErbB2, Akt, ERK and β-catenin along with decreased β-catenin transcriptional activity as measured by c-myc and axin2 expression. Upregulation of RTK or WNT signals are frequent oncogenic events in CRC and many other cancers. Thus, in addition to the role of Hsp70 in cell-survival after transformation, Hsp70 stabilization of β-catenin, Akt, ERK and ErbB2 are predicted to contribute to transformation. This has important implications not only for understanding the pathophysiology of these cancers, but also for treatment since anti-EGFR antibodies are in clinical use for CRC and EGFR is a major ErbB2 heterodimeric partner. Targeting Hsp70, therefore, might provide an alternative or complementary strategy for achieving better outcomes for CRC and other related cancer types.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

