Comprehensive Genomic Profiling of Clinically Advanced Medullary Thyroid Carcinoma
- PMID: 27207748
- PMCID: PMC4909575
- DOI: 10.1159/000445978
Comprehensive Genomic Profiling of Clinically Advanced Medullary Thyroid Carcinoma
Abstract
Objective: The aim of this study was to determine the genomic alterations of cancer-related genes in advanced medullary thyroid carcinoma during the course of clinical care.
Methods: Hybrid-capture-based comprehensive genomic profiling was performed on 34 consecutive medullary thyroid carcinoma cases to identify all four classes of genomic alterations, and outcome for an index patient was collected.
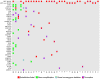
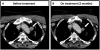
Results: RET was mutated in 88% (30/34) of cases, with RET M918T being responsible for 70% (21/30) of the RET alterations. The other RET alterations were RET E632_L633del, C634R, C620R, C618G/R/S, V804M, and RET amplification. Two of the four RET wild-type patients harbored mutations in KRAS or HRAS (1/34 each). The next most frequent genomic alterations were amplifications of CCND1, FGF3, and FGF19 and alterations in CDKN2A (3/34 each). One case with a RET M918T mutation developed acquired resistance to progressively dose-escalated vandetanib. When the mTOR inhibitor everolimus was added to continued vandetanib treatment, the patient achieved a second 25% reduction of tumor volume (RECIST 1.1) for 8 months.
Conclusions: Comprehensive genomic profiling identified the full breadth of RET alterations in metastatic medullary thyroid carcinoma and possible cooperating oncogenic driver alterations. This approach may refine the use of targeted therapy for these patients.
© 2016 S. Karger AG, Basel.
Figures
References
-
- Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. - PubMed
-
- Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–547. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

