The Rap1-RIAM-talin axis of integrin activation and blood cell function
- PMID: 27207789
- PMCID: PMC4965904
- DOI: 10.1182/blood-2015-12-638700
The Rap1-RIAM-talin axis of integrin activation and blood cell function
Abstract
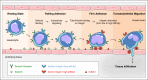
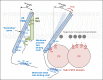
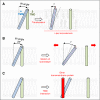
Integrin adhesion receptors mediate the adhesion of blood cells, such as leukocytes, to other cells, such as endothelial cells. Integrins also are critical for anchorage of hematopoietic precursors to the extracellular matrix. Blood cells can dynamically regulate the affinities of integrins for their ligands ("activation"), an event central to their functions. Here we review recent progress in understanding the mechanisms of integrin activation with a focus on the functions of blood cells. We discuss how talin binding to the integrin β cytoplasmic domain, in conjunction with the plasma membrane, induces long-range allosteric rearrangements that lead to integrin activation. Second, we review our understanding of how signaling events, particularly those involving Rap1 small guanosine triphosphate (GTP)hydrolases, can regulate the talin-integrin interaction and resulting activation. Third, we review recent findings that highlight the role of the Rap1-GTP-interacting adapter molecule (RIAM), encoded by the APBB1IP gene, in leukocyte integrin activation and consequently in leukocyte trafficking.
© 2016 by The American Society of Hematology.
Figures

Similar articles
-
Loss of the Rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice.Blood. 2015 Dec 17;126(25):2704-12. doi: 10.1182/blood-2015-05-647453. Epub 2015 Sep 3. Blood. 2015. PMID: 26337492 Free PMC article.
-
Rap1 and membrane lipids cooperatively recruit talin to trigger integrin activation.J Cell Sci. 2019 Nov 1;132(21):jcs235531. doi: 10.1242/jcs.235531. J Cell Sci. 2019. PMID: 31578239 Free PMC article.
-
Two modes of integrin activation form a binary molecular switch in adhesion maturation.Mol Biol Cell. 2013 May;24(9):1354-62. doi: 10.1091/mbc.E12-09-0695. Epub 2013 Mar 6. Mol Biol Cell. 2013. PMID: 23468527 Free PMC article.
-
Integrin activation.Biochem Soc Trans. 2008 Apr;36(Pt 2):229-34. doi: 10.1042/BST0360229. Biochem Soc Trans. 2008. PMID: 18363565 Free PMC article. Review.
-
Molecular mechanisms of leukocyte β2 integrin activation.Blood. 2022 Jun 16;139(24):3480-3492. doi: 10.1182/blood.2021013500. Blood. 2022. PMID: 35167661 Free PMC article. Review.
Cited by
-
Severe bleeding and absent ADP-induced platelet aggregation associated with inherited combined CalDAG-GEFI and P2Y12 deficiencies.Haematologica. 2020 Jul;105(7):e361-e364. doi: 10.3324/haematol.2019.232850. Epub 2019 Oct 24. Haematologica. 2020. PMID: 31649128 Free PMC article. No abstract available.
-
Cyclase-associated protein 1 (CAP1) is a prenyl-binding partner of Rap1 GTPase.J Biol Chem. 2018 May 18;293(20):7659-7673. doi: 10.1074/jbc.RA118.001779. Epub 2018 Apr 4. J Biol Chem. 2018. PMID: 29618512 Free PMC article.
-
Producing highly effective extracellular vesicles using IBAR and talin F3 domain fusion.Anim Cells Syst (Seoul). 2024 May 18;28(1):283-293. doi: 10.1080/19768354.2024.2353159. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 38770055 Free PMC article.
-
Integrin Regulators in Neutrophils.Cells. 2022 Jun 25;11(13):2025. doi: 10.3390/cells11132025. Cells. 2022. PMID: 35805108 Free PMC article. Review.
-
Integrated proteome and malonylome analyses reveal the potential meaning of TLN1 and ACTB in end-stage renal disease.Proteome Sci. 2023 Oct 13;21(1):18. doi: 10.1186/s12953-023-00211-y. Proteome Sci. 2023. PMID: 37833721 Free PMC article.
References
-
- Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15(11):692–704. - PubMed
-
- Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. - PubMed
-
- Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

